The primacy of gastrointestinal tract antigen-presenting cells in lethal graft-versus-host disease
- PMID: 31697827
- PMCID: PMC6908833
- DOI: 10.1182/blood.2019000823
The primacy of gastrointestinal tract antigen-presenting cells in lethal graft-versus-host disease
Abstract
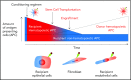
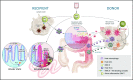
Allogeneic stem cell transplantation is a cornerstone of curative therapy for high-risk and/or advanced hematological malignancies but remains limited by graft-versus-host disease (GVHD). GVHD is initiated by the interaction between recipient antigen-presenting cells (APCs) and donor T cells, culminating in T-cell differentiation along pathogenic type-1 and type-17 paradigms at the expense of tolerogenic regulatory T-cell patterns. Type-1 and type-17 T cells secrete cytokines (eg, granulocyte-macrophage colony-stimulating factor and interferon-γ) critical to the cytokine storm that amplifies expansion of donor APCs and their alloantigen presentation. It has become increasingly clear that pathogenic donor T-cell differentiation is initiated by both professional recipient APCs (eg, dendritic cells [DCs]) and nonprofessional APCs (eg, epithelial and mesenchymal cells), particularly within the gastrointestinal (GI) tract. In the immediate peritransplantation period, these APCs are profoundly modified by pathogen-associated molecular pattern (PAMP)/damage-associated molecular pattern (DAMP) signals derived from conditioning and intestinal microbiota. Subsequently, donor DCs in the GI tract are activated by DAMP/PAMP signals in the colon that gain access to the lamina propria once the mucosal barrier mucosa is compromised by GVHD. This results in donor DC expansion and alloantigen presentation in the colon and subsequent migration into the mesenteric lymph nodes. Here, new donor T cells are primed, expanded, differentiated, and imprinted with gut-homing integrins permissive of migration into the damaged GI tract, resulting in the lethal feed-forward cascade of GVHD. These new insights into our understanding of the cellular and molecular factors initiating GVHD, both spatially and temporally, give rise to a number of logical therapeutic targets, focusing on the inhibition of APC function in the GI tract.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.K. and G.R.H. have submitted a patent application on methods to prevent antigen presentation in the GI tract. G.R.H. has received funding from Roche for a clinical study of tocilizumab in acute GVHD prophylaxis.
Figures

References
-
- Shlomchik WD, Couzens MS, Tang CB, et al. . Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285(5426):412-415. - PubMed
-
- Weiden PL, Flournoy N, Thomas ED, et al. . Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300(19):1068-1073. - PubMed
-
- Marmont AM, Horowitz MM, Gale RP, et al. . T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78(8):2120-2130. - PubMed
-
- Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2017;377(26):2565-2579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

