Human milk-derived fortifier versus bovine milk-derived fortifier for prevention of mortality and morbidity in preterm neonates
- PMID: 31697857
- PMCID: PMC6837687
- DOI: 10.1002/14651858.CD013145.pub2
Human milk-derived fortifier versus bovine milk-derived fortifier for prevention of mortality and morbidity in preterm neonates
Abstract
Background: Preterm infants who are fed breast milk in comparison to infant formula have decreased morbidity such as necrotizing enterocolitis. Multi-nutrient fortifiers used to increase the nutritional content of the breast milk are commonly derived from bovine milk. Human milk-derived multi-nutrient fortifier is now available, but it is not clear if it improves outcomes in preterm infants fed with breast milk.
Objectives: To determine whether the fortification of breast milk feeds with human milk-derived fortifier in preterm infants reduces mortality, morbidity, and promotes growth and development compared to bovine milk-derived fortifier.
Search methods: We searched the following databases for relevant trials in September 2018. Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, Issue 9), electronic journal reference databases including MEDLINE (1980 to 20 September 2018), PREMEDLINE, Embase (1974 to 20 September 2018), CINAHL (1982 to 20 September 2018), biological abstracts in the database BIOSIS and conference abstracts from 'Proceedings First' (from 1992 to 2011). We also included the following clinical trials registries for ongoing or recently completed trials: ClinicalTrials.gov (ClinicalTrials.gov), the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.whoint/ictrp/search/en/) and the ISRCTN Registry (www.isrctn.com/), and abstracts of conferences: proceedings of Pediatric Academic Societies (American Pediatric Society, Society for Pediatric Research and European Society for Paediatric Research) from 1990 in the 'Pediatric Research' journal and 'Abstracts online' (2000 to 2017).
Selection criteria: We included randomized and quasi-randomized controlled trials that compared preterm infants fed breast milk fortified with human milk-derived fortifier versus those fed with breast milk fortified with bovine milk-derived fortifier.
Data collection and analysis: The data were collected using the standard methods of Cochrane Neonatal. Two authors evaluated trial quality of the studies and extracted data. We reported dichotomous data using risk ratios (RRs), risk differences (RDs), number needed to treat (NNT) where applicable, and continuous data using mean differences (MDs). We assessed the quality of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
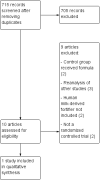
Main results: One randomized trial with 127 infants met the eligibility criteria and had low risk of bias. Human milk-based fortifier did not decrease the risk of necrotizing enterocolitis in exclusively breast milk-fed preterm infants (RR 0.95, 95% CI 0.2 to 4.54; 1 study, 125 infants, low certainty of evidence). Human milk-derived fortifiers did not improve growth, decrease feeding intolerance, late-onset sepsis, or death.
Authors' conclusions: There is insufficient evidence evaluating human milk-derived fortifier with bovine milk-derived fortifier in exclusively breast milk-fed preterm infants. Low-certainty evidence from one study suggests that in exclusively breast milk-fed preterm infants human milk-derived fortifiers in comparison with bovine milk-derived fortifier may not change the risk of necrotizing enterocolitis, mortality, feeding intolerance, infection, or improve growth. Well-designed randomized controlled trials are needed to evaluate short-term and long-term outcomes.
Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Muralidhar H Premkumar has no known conflicts of interest relevant to this review. Mohan Pammi has no known conflicts of interest relevant to this review. Gautham Suresh has no known conflicts of interest relevant to this review.
The methods section of this review is based on a standard template used by Cochrane Neonatal.
Core editorial and administrative support for this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, distinct from the Gerber Products Company. The grantor has no input on the content of the review or the editorial process.
In order to maintain the utmost editorial independence for this Cochrane Review, an editor outside of the Cochrane Neonatal core editorial team who is not receiving any financial remuneration from the grant, William McGuire, was the Sign‐off Editor for this review. Additionally, a Senior Editor from the Cochrane Children and Families Network, Robert Boyle, assessed and signed off on this Cochrane Review.
Figures
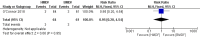
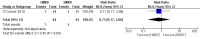
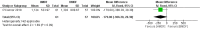

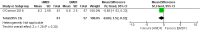


Update of
- doi: 10.1002/14651858.CD013145
References
References to studies included in this review
O'Connor 2018 {published data only}
-
- O'Connor DL, Kiss A, Tomlinson C, Brando N, Bayliss A, Campbell DM, et al. OptiMoM Feeding Group. Nutrient enrichment of human milk with human and bovine milk–based fortifiers for infants born weighing <1250 g: a randomized clinical trial. American Journal of Clinical Nutrition 2018;108(1):108‐16. [DOI: 10.1093/ajcn/nqy067; PUBMED: 29878061] - DOI - PubMed
References to studies excluded from this review
Abrams 2014 {published data only}
Adhisivam 2018 {published data only}
-
- Adhisivam B, Kohat D, Tanigasalam V, Bhat V, Plakkal N, Palanivel C. Does fortification of pasteurized donor human milk increase the incidence of necrotizing enterocolitis among preterm neonates? A randomized controlled trial. Journal of Maternal‐Fetal & Neonatal Medicine 2018;18(18):1‐6. [DOI: 10.1080/14767058.2018.1461828; PUBMED: 29618272] - DOI - PubMed
Assad 2016 {published data only}
Colacci 2017 {published data only}
Corpeleijn 2016 {published data only}
-
- Corpeleijn WE, Waard M, Christmann V, Goudoever JB, Jansen‐Van der Weide MC, Kooi EM, et al. Effect of donor milk on severe infections and mortality in very low‐birth‐weight infants: the early nutrition study randomized clinical trial. JAMA Pediatrics 2016;170(7):654‐61. [DOI: 10.1001/jamapediatrics.2016.0183; PUBMED: 27135598] - DOI - PubMed
Cristofalo 2013 {published data only}
-
- Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl‐Kohlendorfer U, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. Journal of Pediatrics 2013;163(6):1592‐5. [DOI: 10.1016/j.jpeds.2013.07.011; PUBMED: 23968744] - DOI - PubMed
Ganapathy 2012 {published data only}
Ghandehari 2012 {published data only}
-
- Ghandehari H, Lee ML, Rechtman DJ, H2MF Study Group. An exclusive human milk‐based diet in extremely premature infants reduces the probability of remaining on total parenteral nutrition: a reanalysis of the data. BMC Research Notes 2012;5(188):1‐5. [DOI: 10.1186/1756-0500-5-188; PUBMED: 22534258] - DOI - PMC - PubMed
Sullivan 2010 {published data only}
-
- Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawoger R, Kiechl‐Kohlendorfer U, et al. An exclusively human milk‐based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk‐based products. Journal of Pediatrics 2010;156(4):562‐7. [DOI: 10.1016/j.jpeds.2009.10.040; PUBMED: 20036378] - DOI - PubMed
Additional references
AAP 2012
Abrams 2013
Agostini 2010
-
- Agostoni C, Buonocore G, Carnielli VP, Curtis M, Darmaun D, Decsi T, et al. ESPGHAN Committee on Nutrition. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology Nutrition 2010;50(1):85‐91. [DOI: 10.1097/MPG.0b013e3181adaee0; PUBMED: 19881390] - DOI - PubMed
Bayley 1993
-
- Bayley N. Bayley Scales of Infant Development. 2nd Edition. London (UK): Pearson, 1993.
Bayley 2005
-
- Bayley N. Bayley Scales of Infant Development. 3rd Edition. London (UK): Pearson, 2005.
Blencowe 2012
-
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379(9832):2162‐72. [DOI: 10.1016/S0140-6736(12)60820-4; PUBMED: 22682464] - DOI - PubMed
Brown 2016
Dutta 2015
Embleton 2007
Embleton 2017
Gathwala 2007
-
- Gathwala G, Chawla M, Gehlaut VS. Fortified human milk in the small for gestational age neonate. Indian Journal of Pediatrics 2007;74(9):815‐8. [PUBMED: 17901665] - PubMed
Gephart 2012
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 16 October 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2017
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
Horbar 2012
Hsiao 2014
ICROP 2005
Jobe 2001
Lager 2012
Lönnerdal 2017
Maas 2018
McNelis 2017
Mimouni 2017
Mitchell 2009
-
- Mitchell SM, Rogers SP, Hicks PD, Hawthorne KM, Parker BR, Abrams SA. High frequencies of elevated alkaline phosphatase activity and rickets exist in extremely low birth weight infants despite current nutritional support. BMC Pediatrics 2009;9:47. [DOI: 10.1186/1471-2431-9-47; PUBMED: 19640269] - DOI - PMC - PubMed
Moher 2009
Moore 2011
Mukhopadhyay 2007
-
- Mukhopadhyay K, Narnag A, Mahajan R. Effect of human milk fortification in appropriate for gestation and small for gestation preterm babies: a randomized controlled trial. Indian Pediatrics 2007;44(4):286‐90. [PUBMED: 17468524] - PubMed
NCHS 2018
-
- National Center for Health Statistics. National Center for Health Statistics, final natality data. www.marchofdimes.org/peristats/Peristats.aspx (accessed 3 October 2018).
Nehra 2014
-
- Nehra D, Fallon EM, Potemkin AK, Voss SD, Mitchell PD, Valim C, et al. A comparison of 2 intravenous lipid emulsions: interim analysis of a randomized controlled trial. Journal of Parenteral and Enteral Nutrition 2014;38(6):693‐701. [DOI: 10.1177/0148607113492549; PUBMED: 23770843] - DOI - PMC - PubMed
Polberger 1989
Quigley 2018
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s), GRADE Working Group. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed prior to 23 September 2019).
Sisk 2007
Sisk 2017
-
- Sisk PM, Lambeth TM, Rojas MA, Lightbourne T, Barahona M, Anthony E, et al. Necrotizing enterocolitis and growth in preterm infants fed predominantly maternal milk, pasteurized donor milk, or preterm formula: a retrospective study. American Journal of Perinatology 2017;34(7):676‐83. [DOI: 10.1055/s-0036-1597326; PUBMED: 27936476] - DOI - PubMed
Skouteris 2017
Tudehope 2013
Walsh 1986
WHO 2017
-
- World Health Organization. WHO fact sheet. 2017. www.who.int/mediacentre/factsheets/fs363/en (accessed 25 February 2018).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

