Oxysterols as lipid mediators: Their biosynthetic genes, enzymes and metabolites
- PMID: 31698146
- PMCID: PMC7081179
- DOI: 10.1016/j.prostaglandins.2019.106381
Oxysterols as lipid mediators: Their biosynthetic genes, enzymes and metabolites
Abstract
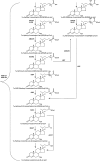
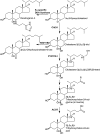
There is growing evidence that oxysterols are more than simple metabolites in the pathway from cholesterol to bile acids. Recent data has shown oxysterols to be ligands to nuclear receptors and to G protein-coupled receptors, modulators of N-methyl-d-aspartate receptors and regulators of cholesterol biosynthesis. In this mini-review we will discuss the biosynthetic mechanisms for the formation of different oxysterols and the implication of disruption of these mechanisms in health and disease.
Keywords: Dihydroxycholesterol; Epoxycholesterol; Epstein Barr virus induced gene 2; G protein-coupled receptor; Hedgehog signaling; Hydroxycholesterol; Liver X receptor; Nuclear receptor; Smoothened.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Schroepfer G.J., Jr. Oxysterols: modulators of cholesterol metabolism and other processes. Physiol. Rev. 2000;80(1):361–554. - PubMed
-
- Javitt N.B. Oxysteroids: a new class of steroids with autocrine and paracrine functions. Trends Endocrinol. Metab. 2004;15(8):393–397. - PubMed
-
- Lehmann J.M., Kliewer S.A., Moore L.B., Smith-Oliver T.A., Oliver B.B., Su J.L., Sundseth S.S., Winegar D.A., Blanchard D.E., Spencer T.A., Willson T.M. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 1997;272(6):3137–3140. - PubMed
-
- Umetani M., Domoto H., Gormley A.K., Yuhanna I.S., Cummins C.L., Javitt N.B., Korach K.S., Shaul P.W., Mangelsdorf D.J. 27-hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat. Med. 2007;13(10):1185–1192. - PubMed
-
- Soroosh P., Wu J., Xue X., Song J., Sutton S.W., Sablad M., Yu J., Nelen M.I., Liu X., Castro G., Luna R., Crawford S., Banie H., Dandridge R.A., Deng X., Bittner A., Kuei C., Tootoonchi M., Rozenkrants N., Herman K., Gao J., Yang X.V., Sachen K., Ngo K., Fung-Leung W.P., Nguyen S., de Leon-Tabaldo A., Blevitt J., Zhang Y., Cummings M.D., Rao T., Mani N.S., Liu C., McKinnon M., Milla M.E., Fourie A.M., Sun S. Oxysterols are agonist ligands of RORgammat and drive Th17 cell differentiation. Proc. Natl. Acad. Sci. U. S. A. 2014;111(33):12163–12168. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

