The Synthesis and Utility of Metal-Nitrosophenolato Compounds-Highlighting the Baudisch Reaction
- PMID: 31698829
- PMCID: PMC6891451
- DOI: 10.3390/molecules24224018
The Synthesis and Utility of Metal-Nitrosophenolato Compounds-Highlighting the Baudisch Reaction
Abstract
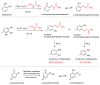
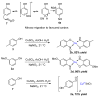
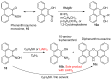
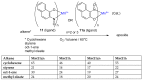
The syntheses of the title compounds demonstrate a privileged introduction of a nitroso (and a hydroxyl via the Baudisch reaction) group to an aromatic ring. These complexes first appeared in the literature as early as 1939, and a range of applications has subsequently been published. However, optimisations of the preparative sequences were not considered, and as such, the reactions have seldom been utilised in recent years; indeed, there remains confusion in the literature as to how such complexes form. In this review, we aim to demystify the misunderstanding surrounding these remarkable complexes and consider their renewed application in the 21st century.
Keywords: Baudisch reaction; C-nitrosation; Copper complexes; ortho-nitrosophenols.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript or in the decision to publish the review.
Figures











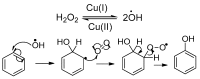



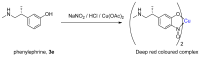


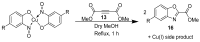
References
-
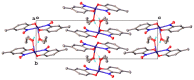
- Charalambous J., Frazer M.J., Taylor F.B. Complexes of copper(II) with o-nitrosophenols (monoximes of ortho-benzoquinones) J. Chem. Soc. A Inorg. Phys. Theor. 1969:2787. doi: 10.1039/j19690002787. - DOI
-
- Al-Obaidi U., Moodie R.B. The nitrous acid-catalysed nitration of phenol. J. Chem. Soc. Perkin Trans. 2. 1985:467. doi: 10.1039/p29850000467. - DOI
-
- Zhang M. Production Device of Rubber Colorant Pigment Green B 2017. [(accessed on 28 July 2019)]; Available online: https://patents.google.com/patent/CN108239422A/en?oq=CN108239422A.
-
- Wegener J.W., Klamer J.C., Govers H., Brinkman U.A.T. Determination of organic colorants in cosmetic products by high-performance liquid chromatography. Chromatographia. 1987;24:865–875. doi: 10.1007/BF02688601. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

