FGF23 and Phosphate-Cardiovascular Toxins in CKD
- PMID: 31698866
- PMCID: PMC6891626
- DOI: 10.3390/toxins11110647
FGF23 and Phosphate-Cardiovascular Toxins in CKD
Abstract
Elevated levels of fibroblast growth factor 23 (FGF23) and phosphate are highly associated with increased cardiovascular disease and mortality in patients suffering from chronic kidney disease (CKD). As the kidney function declines, serum phosphate levels rise and subsequently induce the secretion of the phosphaturic hormone FGF23. In early stages of CKD, FGF23 prevents the increase of serum phosphate levels and thereby attenuates phosphate-induced vascular calcification, whereas in end-stage kidney disease, FGF23 fails to maintain phosphate homeostasis. Both hyperphosphatemia and elevated FGF23 levels promote the development of hypertension, vascular calcification, and left ventricular hypertrophy by distinct mechanisms. Therefore, FGF23 and phosphate are considered promising therapeutic targets to improve the cardiovascular outcome in CKD patients. Previous therapeutic strategies are based on dietary and pharmacological reduction of serum phosphate, and consequently FGF23 levels. However, clinical trials proving the effects on the cardiovascular outcome are lacking. Recent publications provide evidence for new promising therapeutic interventions, such as magnesium supplementation and direct targeting of phosphate and FGF receptors to prevent toxicity of FGF23 and hyperphosphatemia in CKD patients.
Keywords: FGF23; cardiovascular disease; chronic kidney disease; hypertension; left ventricular hypertrophy; phosphate; vascular calcification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


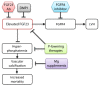
References
-
- Isakova T., Wahl P., Vargas G.S., Gutiérrez O.M., Scialla J., Xie H., Appleby D., Nessel L., Bellovich K., Chen J., et al. Fibroblast Growth Factor 23 is Elevated before Parathyroid Hormone and Phosphate in Chronic Kidney Disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. - DOI - PMC - PubMed
-
- Palmer S.C., Hayen A., Macaskill P., Pellegrini F., Craig J.C., Elder G.J., Strippoli G.F.M. Serum Levels of Phosphorus, Parathyroid Hormone, and Calcium and Risks of Death and Cardiovascular Disease in Individuals with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. JAMA. 2011;305:1119–1127. doi: 10.1001/jama.2011.308. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

