PTH/PTHrP Receptor Signaling, Allostery, and Structures
- PMID: 31699241
- PMCID: PMC6857722
- DOI: 10.1016/j.tem.2019.07.011
PTH/PTHrP Receptor Signaling, Allostery, and Structures
Abstract
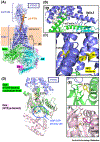
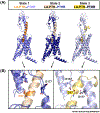
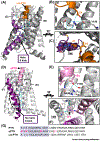
The parathyroid hormone (PTH) type 1 receptor (PTHR) is the canonical G protein-coupled receptor (GPCR) for PTH and PTH-related protein (PTHrP) and the key regulator of calcium homeostasis and bone turnover. PTHR function is critical for human health to maintain homeostatic control of ionized serum Ca2+ levels and has several unusual signaling features, such as endosomal cAMP signaling, that are well-studied but not structurally understood. In this review, we discuss how recently solved high resolution near-atomic structures of hormone-bound PTHR in its inactive and active signaling states and discovery of extracellular Ca2+ allosterism shed light on the structural basis for PTHR signaling and function.
Keywords: G protein-coupled receptor; allostery; cAMP; cryo-EM structure; endosomes; parathyroid hormone.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures


References
-
- Ureña P et al. (1993) Parathyroid hormone (PTH)/PTH-related peptide receptor messenger ribonucleic acids are widely distributed in rat tissues. Endocrinology 133, 617–623 - PubMed
-
- Horwitz MJ et al. (2003) Short-term, high-dose parathyroid hormone-related protein as a skeletal anabolic agent for the treatment of postmenopausal osteoporosis. J. Clin. Endocrinol. Metab 88, 569–575 - PubMed
-
- Horwitz MJ et al. (2003) Direct comparison of sustained infusion of human parathyroid hormone-related protein-(1-36) [hPTHrP-(1-36)] versus hPTH-(1-34) on serum calcium, plasma 1,25-dihydroxyvitamin D concentrations, and fractional calcium excretion in healthy human volunteers. J. Clin. Endocrinol. Metab 88, 1603–1609 - PubMed
-
- Horwitz MJ et al. (2005) Continuous PTH and PTHrP infusion causes suppression of bone formation and discordant effects on 1,25(OH)2 vitamin D. J. Bone Miner. Res 20, 1792–1803 - PubMed
-
- Neer RM et al. (2001) Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med 344,1434–1441 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

