Convergent Evidence for Predispositional Effects of Brain Gray Matter Volume on Alcohol Consumption
- PMID: 31699293
- PMCID: PMC7412715
- DOI: 10.1016/j.biopsych.2019.08.029
Convergent Evidence for Predispositional Effects of Brain Gray Matter Volume on Alcohol Consumption
Abstract
Background: Alcohol use has been reliably associated with smaller subcortical and cortical regional gray matter volumes (GMVs). Whether these associations reflect shared predisposing risk factors or causal consequences of alcohol use remains poorly understood.
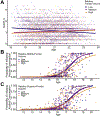
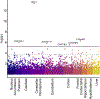
Methods: Data came from 3 neuroimaging samples (N = 2423), spanning childhood or adolescence to middle age, with prospective or family-based data. First, we identified replicable GMV correlates of alcohol use. Next, we used family-based and longitudinal data to test whether these associations may plausibly reflect a predispositional liability for alcohol use or a causal consequence of alcohol use. Finally, we used heritability, gene-set enrichment, and transcriptome-wide association study approaches to evaluate whether genome-wide association study-defined genomic risk for alcohol consumption is enriched for genes that are preferentially expressed in regions that were identified in our neuroimaging analyses.
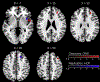
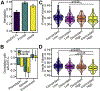
Results: Smaller right dorsolateral prefrontal cortex (DLPFC) (i.e., middle and superior frontal gyri) and insula GMVs were associated with increased alcohol use across samples. Family-based and prospective longitudinal data suggest that these associations are genetically conferred and that DLPFC GMV prospectively predicts future use and initiation. Genomic risk for alcohol use was enriched in gene sets that were preferentially expressed in the DLPFC and was associated with replicable differential gene expression in the DLPFC.
Conclusions: These data suggest that smaller DLPFC and insula GMV plausibly represent genetically conferred predispositional risk factors for, as opposed to consequences of, alcohol use. DLPFC and insula GMV represent promising biomarkers for alcohol-consumption liability and related psychiatric and behavioral phenotypes.
Keywords: Alcohol; Gene expression; Heritability; Imaging; Longitudinal; Structure.
Copyright © 2019 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- World Health Organization (2014): Global status report on alcohol and health. Geneva, Switzerland: World Health Organization Press.
-
- Substance Abuse and Mental Health Services Administration (2018): Key Substance Use and Mental Health Indicators in the United States: Results from the 2017 National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; Available at: https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHFFR20.... Accessed February 22, 2018.
-
- Lange EHH, Nerland S, Jørgensen KNN, Mørch-Johnsen L, Nesvåg R, Hartberg CBB, et al. (2017): Alcohol use is associated with thinner cerebral cortex and larger ventricles in schizophrenia, bipolar disorder and healthy controls. Psychol Med 4:55–668. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AG049789/AG/NIA NIH HHS/United States
- K23 AA025399/AA/NIAAA NIH HHS/United States
- R01 DA031579/DA/NIDA NIH HHS/United States
- U01 AG052564/AG/NIA NIH HHS/United States
- T32 DA007313/DA/NIDA NIH HHS/United States
- U54 MH091657/MH/NIMH NIH HHS/United States
- R01 AG045231/AG/NIA NIH HHS/United States
- T32 GM081739/GM/NIGMS NIH HHS/United States
- R01 HD083614/HD/NICHD NIH HHS/United States
- R01 EY012135/EY/NEI NIH HHS/United States
- R01 AA016274/AA/NIAAA NIH HHS/United States
- R01 AG061162/AG/NIA NIH HHS/United States
- K02 DA032573/DA/NIDA NIH HHS/United States
- T32 GM008151/GM/NIGMS NIH HHS/United States
- R01 DA033369/DA/NIDA NIH HHS/United States
- F32 AA023693/AA/NIAAA NIH HHS/United States
- U01 DA041093/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources

