Microbial Control of Intestinal Homeostasis via Enteroendocrine Cell Innate Immune Signaling
- PMID: 31699645
- PMCID: PMC6980660
- DOI: 10.1016/j.tim.2019.09.005
Microbial Control of Intestinal Homeostasis via Enteroendocrine Cell Innate Immune Signaling
Abstract
A community of commensal microbes, known as the intestinal microbiota, resides within the gastrointestinal tract of animals and plays a role in maintenance of host metabolic homeostasis and resistance to pathogen invasion. Enteroendocrine cells, which are relatively rare in the intestinal epithelium, have evolved to sense and respond to these commensal microbes. Specifically, they express G-protein-coupled receptors and functional innate immune signaling pathways that recognize products of microbial metabolism and microbe-associated molecular patterns, respectively. Here we review recent evidence from Drosophila melanogaster that microbial cues recruit antimicrobial, mechanical, and metabolic branches of the enteroendocrine innate immune system and argue that this response may play a role not only in maintaining host metabolic homeostasis but also in intestinal resistance to invasion by bacterial, viral, and parasitic pathogens.
Keywords: Drosophila melanogaster.; colonization resistance; enteroendocrine cell; enteroendocrine peptide; innate immunity; intestinal microbiota; metabolism.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures
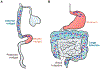
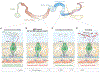
Similar articles
-
The Drosophila Immune Deficiency Pathway Modulates Enteroendocrine Function and Host Metabolism.Cell Metab. 2018 Sep 4;28(3):449-462.e5. doi: 10.1016/j.cmet.2018.05.026. Epub 2018 Jun 21. Cell Metab. 2018. PMID: 29937377 Free PMC article.
-
Microbiota-derived acetate activates intestinal innate immunity via the Tip60 histone acetyltransferase complex.Immunity. 2021 Aug 10;54(8):1683-1697.e3. doi: 10.1016/j.immuni.2021.05.017. Epub 2021 Jun 8. Immunity. 2021. PMID: 34107298 Free PMC article.
-
The interplay between intestinal bacteria and host metabolism in health and disease: lessons from Drosophila melanogaster.Dis Model Mech. 2016 Mar;9(3):271-81. doi: 10.1242/dmm.023408. Dis Model Mech. 2016. PMID: 26935105 Free PMC article. Review.
-
Enteroendocrine Cells: Sensing Gut Microbiota and Regulating Inflammatory Bowel Diseases.Inflamm Bowel Dis. 2020 Jan 1;26(1):11-20. doi: 10.1093/ibd/izz217. Inflamm Bowel Dis. 2020. PMID: 31560044 Free PMC article. Review.
-
Drosophila as a model for intestinal dysbiosis and chronic inflammatory diseases.Dev Comp Immunol. 2014 Jan;42(1):102-10. doi: 10.1016/j.dci.2013.05.005. Epub 2013 May 16. Dev Comp Immunol. 2014. PMID: 23685204 Review.
Cited by
-
Inflammaging: The Next Challenge-Exploring the Role of Gut Microbiota, Environmental Factors, and Sex Differences.Biomedicines. 2024 Aug 1;12(8):1716. doi: 10.3390/biomedicines12081716. Biomedicines. 2024. PMID: 39200181 Free PMC article. Review.
-
Methionine Availability in the Arthropod Intestine Is Elucidated through Identification of Vibrio cholerae Methionine Acquisition Systems.Appl Environ Microbiol. 2020 May 19;86(11):e00371-20. doi: 10.1128/AEM.00371-20. Print 2020 May 19. Appl Environ Microbiol. 2020. PMID: 32220836 Free PMC article.
-
Gastrointestinal acute radiation syndrome: current knowledge and perspectives.Cell Death Discov. 2025 May 14;11(1):235. doi: 10.1038/s41420-025-02525-6. Cell Death Discov. 2025. PMID: 40368913 Free PMC article. Review.
-
Drosophila: An Important Model for Exploring the Pathways of Inflammatory Bowel Disease (IBD) in the Intestinal Tract.Int J Mol Sci. 2024 Nov 27;25(23):12742. doi: 10.3390/ijms252312742. Int J Mol Sci. 2024. PMID: 39684456 Free PMC article. Review.
-
Csf1r mediates enhancement of intestinal tumorigenesis caused by inactivation of Mir34a.Int J Biol Sci. 2022 Aug 29;18(14):5415-5437. doi: 10.7150/ijbs.75503. eCollection 2022. Int J Biol Sci. 2022. PMID: 36147476 Free PMC article.
References
-
- Dutta D et al. (2015) Regional Cell-Specific Transcriptome Mapping Reveals Regulatory Complexity in the Adult Drosophila Midgut. Cell Rep 12 (2), 346–58. - PubMed
-
- Buchon N et al. (2013) Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep 3 (5), 1725–38. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

