CRISTAL: protocol for a cluster randomised, crossover, non-inferiority trial of aspirin compared to low molecular weight heparin for venous thromboembolism prophylaxis in hip or knee arthroplasty, a registry nested study
- PMID: 31699735
- PMCID: PMC6858170
- DOI: 10.1136/bmjopen-2019-031657
CRISTAL: protocol for a cluster randomised, crossover, non-inferiority trial of aspirin compared to low molecular weight heparin for venous thromboembolism prophylaxis in hip or knee arthroplasty, a registry nested study
Erratum in
-
Correction: CRISTAL: protocol for a cluster randomised, crossover, non-inferiority trial of aspirin compared to low molecular weight heparin for venous thromboembolism prophylaxis in hip or knee arthroplasty, a registry nested study.BMJ Open. 2022 Mar 29;12(3):e031657corr1. doi: 10.1136/bmjopen-2019-031657corr1. BMJ Open. 2022. PMID: 35351736 Free PMC article. No abstract available.
Abstract
Introduction: Venous thromboembolism (VTE) is a serious complication following hip arthroplasty (HA) and knee arthroplasty (KA). This study aims to determine whether aspirin is non-inferior to low molecular weight heparin (LMWH) in preventing symptomatic VTE following HA and KA.
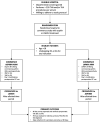
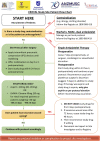
Methods and analysis: This is a cluster randomised, crossover, non-inferiority, trial nested within the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). The clusters will consist of Australian hospitals performing at least 250 HA and/or KA procedures per annum. All adult patients undergoing HA or KA will be included. The intervention will be aspirin, orally, 85-150 mg daily. The comparator will be LMWH (enoxaparin) 40 mg, subcutaneously, daily. Both drugs will commence within 24 hours postoperatively and continue for 35 days after HA and 14 days after KA. Each hospital will be randomised to commence with aspirin or LMWH and then crossover to the alternative treatment after meeting the recruitment target. Data will be collected through the AOANJRR via patient-reported surveys. The primary outcome is symptomatic VTE within 90 days post surgery, verified by AOANJRR staff. The primary analysis will include only patients undergoing elective primary total hip arthroplasty and total knee arthroplasty for osteoarthritis. Secondary outcomes will include symptomatic VTE for all HA and KA (including partial and revision) within 90 days, readmission, reoperation, major bleeding and death within 90 days and reoperation, death and patient-reported pain, function and health status at 6 months. If aspirin is found to be inferior, a cost-effectiveness analysis will be conducted. The study will aim to recruit 15 562 patients from 31 hospitals.
Ethics and dissemination: Ethics approval has been granted. Trial results will be submitted for publication. The trial is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12618001879257, pre-results) and is endorsed by the Australia and New Zealand Musculoskeletal Clinical Trials Network.
Keywords: aspirin; deep venous thrombosis; hip arthroplasty; knee arthroplasty; low molecular weight heparin; venous thromboembolism.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- AOA . Australian Orthopaedic Association national joint replacement registry annual report, 2017.
-
- Falck-Ytter Y, Francis CW, Johanson NA, et al. . Prevention of VTe in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ED: American College of chest physicians evidence-based clinical practice guidelines. Chest 2012;141:e278S–325. 10.1378/chest.11-2404 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
