Genetic Ablation of Calcium-independent Phospholipase A2γ Exacerbates Glomerular Injury in Adriamycin Nephrosis in Mice
- PMID: 31700134
- PMCID: PMC6838178
- DOI: 10.1038/s41598-019-52834-x
Genetic Ablation of Calcium-independent Phospholipase A2γ Exacerbates Glomerular Injury in Adriamycin Nephrosis in Mice
Abstract
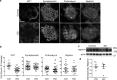
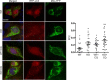
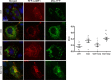
Genetic ablation of calcium-independent phospholipase A2γ (iPLA2γ) in mice results in marked damage of mitochondria and enhanced autophagy in glomerular visceral epithelial cells (GECs) or podocytes. The present study addresses the role of iPLA2γ in glomerular injury. In adriamycin nephrosis, deletion of iPLA2γ exacerbated albuminuria and reduced podocyte number. Glomerular LC3-II increased and p62 decreased in adriamycin-treated iPLA2γ knockout (KO) mice, compared with treated control, in keeping with increased autophagy in KO. iPLA2γ KO GECs in culture also demonstrated increased autophagy, compared with control GECs. iPLA2γ KO GECs showed a reduced oxygen consumption rate and increased phosphorylation of AMP kinase (pAMPK), consistent with mitochondrial dysfunction. Adriamycin further stimulated pAMPK and autophagy. After co-transfection of GECs with mito-YFP (to label mitochondria) and RFP-LC3 (to label autophagosomes), or RFP-LAMP1 (to label lysosomes), there was greater colocalization of mito-YFP with RFP-LC3-II and with RFP-LAMP1 in iPLA2γ KO GECs, compared with WT, indicating enhanced mitophagy in KO. Adriamycin increased mitophagy in WT cells. Thus, iPLA2γ has a cytoprotective function in the normal glomerulus and in glomerulopathy, as deletion of iPLA2γ leads to mitochondrial damage and impaired energy homeostasis, as well as autophagy and mitophagy.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

