Differential expression of CCR2 and CX3CR1 on CD16+ monocyte subsets is associated with asthma severity
- PMID: 31700522
- PMCID: PMC6829828
- DOI: 10.1186/s13223-019-0379-5
Differential expression of CCR2 and CX3CR1 on CD16+ monocyte subsets is associated with asthma severity
Abstract
Background: Monocytes play an important role in immune and inflammatory diseases and monocyte subsets are predictors of disease in certain conditions. Expression of the chemokine receptors, CCR2 and CX3CR1 on monocyte subsets relates to their function and can be used in their characterization. Our objective was to determine whether CD14, CD16, CCR2 and CX3CR1 on monocyte subsets are potential indicators of asthma severity.
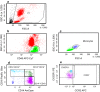
Methods: Blood samples were collected from Saudi Arabian patients with asthma and normal healthy individuals. Six-color flow-cytometry phenotypic analysis was used to identify human blood monocyte subsets, based on their expression of CD14 and CD16 following CD45 gating. Expression of CCR2 and CX3CR1 was analysed on classical (CD14++CD16-), intermediate (CD14++CD16+) and non-classical (CD14+CD16++) subsets and correlated with disease severity.
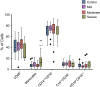
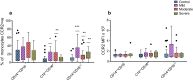
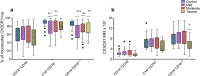
Results: We demonstrated a significant increase in percentage of total CD45-positive monocytes in the blood of patients with severe asthma, but the proportion of the individual monocyte subsets was not significantly changed when patients with mild, moderate and severe asthma were compared with healthy individuals. CD16 expression (mean fluorescence intensity, MFI) was decreased on intermediate and non-classical subsets in patients with severe asthma compared to healthy controls. CX3CR1 expression was also lower, with a lower percentage of cells expressing CX3CR1 in the non-classical CD14+CD16++ subset in all patients with asthma and this was inversely related to the percentage of cells expressing CCR2.
Conclusions: CCR2 expression on monocytes indicated a tendency toward more phagocytic monocytes in patients with asthma. The differential expression of CD16, CX3CR1 and CCR2 on monocyte subsets in peripheral blood indicates modulation of the inflammatory response and suggests a role for monocytes in asthma pathogenesis.
Keywords: Biomarker; CD14; Chemokine receptor; Flow cytometry; Saudi Arabia.
© The Author(s) 2019.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

