Sequential infection can decrease virulence in a fish-bacterium-fluke interaction: Implications for aquaculture disease management
- PMID: 31700534
- PMCID: PMC6824072
- DOI: 10.1111/eva.12850
Sequential infection can decrease virulence in a fish-bacterium-fluke interaction: Implications for aquaculture disease management
Abstract
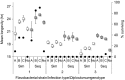
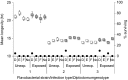
Hosts are typically infected with multiple strains or genotypes of one or several parasite species. These infections can take place simultaneously, but also at different times, i.e. sequentially, when one of the parasites establishes first. Sequential parasite dynamics are common in nature, but also in intensive farming units such as aquaculture. However, knowledge of effects of previous exposures on virulence of current infections in intensive farming is very limited. This is critical as consecutive epidemics and infection history of a host could underlie failures in management practices and medical intervention of diseases. Here, we explored effects of timing of multiple infections on virulence in two common aquaculture parasites, the bacterium Flavobacterium columnare and the fluke Diplostomum pseudospathaceum. We exposed fish hosts first to flukes and then to bacteria in two separate experiments, altering timing between the infections from few hours to several weeks. We found that both short-term and long-term differences in timing of the two infections resulted in significant, genotype-specific decrease in bacterial virulence. Second, we developed a mathematical model, parameterized from our experimental results, to predict the implications of sequential infections for epidemiological progression of the disease, and levels of fish population suppression, in an aquaculture setting. Predictions of the model showed that sequential exposure of hosts can decrease the population-level impact of the bacterial epidemic, primarily through the increased recovery rate of sequentially infected hosts, thereby substantially protecting the population from the detrimental impact of infection. However, these effects depended on bacterial strain-fluke genotype combinations, suggesting the genetic composition of the parasite populations can greatly influence the degree of host suppression. Overall, these results suggest that host infection history can have significant consequences for the impact of infection at host population level, potentially shaping parasite epidemiology, disease dynamics and evolution of virulence in farming environments.
Keywords: dynamic infection; epidemiology; multiple infections; sequential infection; spatiotemporal variation.
© 2019 The Authors. Evolutionary Applications published by John Wiley & Sons Ltd.
Conflict of interest statement
We have no competing interests.
Figures


Similar articles
-
Minor environmental concentrations of antibiotics can modify bacterial virulence in co-infection with a non-targeted parasite.Biol Lett. 2018 Dec 21;14(12):20180663. doi: 10.1098/rsbl.2018.0663. Biol Lett. 2018. PMID: 30958249 Free PMC article.
-
Host infection history modifies co-infection success of multiple parasite genotypes.J Anim Ecol. 2016 Mar;85(2):591-7. doi: 10.1111/1365-2656.12472. Epub 2016 Jan 11. J Anim Ecol. 2016. PMID: 26589834
-
Phage-driven loss of virulence in a fish pathogenic bacterium.PLoS One. 2012 Dec 20;7(12):e53157. doi: 10.1371/journal.pone.0053157. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23308090 Free PMC article.
-
Importance of Sequence and Timing in Parasite Coinfections.Trends Parasitol. 2019 Feb;35(2):109-118. doi: 10.1016/j.pt.2018.11.007. Epub 2018 Dec 18. Trends Parasitol. 2019. PMID: 30578150 Review.
-
The ecology of fish parasites with particular reference to helminth parasites and their salmonid fish hosts in Welsh rivers: a review of some of the central questions.Adv Parasitol. 2002;52:1-154. doi: 10.1016/s0065-308x(02)52011-x. Adv Parasitol. 2002. PMID: 12521260 Review.
Cited by
-
Strategies for managing marine disease.Ecol Appl. 2022 Oct;32(7):e2643. doi: 10.1002/eap.2643. Epub 2022 Jul 21. Ecol Appl. 2022. PMID: 35470930 Free PMC article.
-
An Antioxidant Supplement Function Exploration: Rescue of Intestinal Structure Injury by Mannan Oligosaccharides after Aeromonas hydrophila Infection in Grass Carp (Ctenopharyngodon idella).Antioxidants (Basel). 2022 Apr 20;11(5):806. doi: 10.3390/antiox11050806. Antioxidants (Basel). 2022. PMID: 35624670 Free PMC article.
References
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

