By protecting against cutaneous inflammation, epidermal pigmentation provided an additional advantage for ancestral humans
- PMID: 31700538
- PMCID: PMC6824065
- DOI: 10.1111/eva.12858
By protecting against cutaneous inflammation, epidermal pigmentation provided an additional advantage for ancestral humans
Abstract
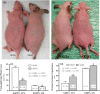
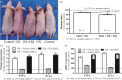
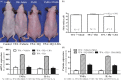
Pigmentation evolved in ancestral humans to protect against toxic, ultraviolet B irradiation, but the question remains: "what is being protected?" Because humans with dark pigmentation display a suite of superior epidermal functions in comparison with their more lightly pigmented counterparts, we hypothesized and provided evidence that dark pigmentation evolved in Africa to support cutaneous function. Because our prior clinical studies also showed that a restoration of a competent barrier dampens cutaneous inflammation, we hypothesized that resistance to inflammation could have provided pigmented hominins with yet another, important evolutionary benefit. We addressed this issue here in two closely related strains of hairless mice, endowed with either moderate (Skh2/J) or absent (Skh1) pigmentation. In these models, we showed that (a) pigmented mice display a markedly reduced propensity to develop inflammation after challenges with either a topical irritant or allergen in comparison with their nonpigmented counterparts; (b) visible and histologic evidence of inflammation was paralleled by reduced levels of pro-inflammatory cytokines (i.e., IL-1α and INFα); (c) because depigmentation of Skh2/J mouse skin enhanced both visible inflammation and pro-inflammatory cytokine levels after comparable pro-inflammatory challenges, the reduced propensity to develop inflammation was directly linked to the presence of pigmentation; and (d) furthermore, in accordance with our prior work showing that pigment production endows benefits by reducing the surface pH of skin, acidification of albino (Skh1) mouse skin also protected against inflammation, and equalized cytokine levels to those found in pigmented skin. In summary, pigmentation yields a reduced propensity to develop inflammation, consistent with our hypothesis that dark pigmentation evolved in ancestral humans to provide a suite of barrier-linked benefits that now include resistance to inflammation.
Keywords: barrier function; epidermis; evolution; inflammation; melanin; pH; pigmentation.
© 2019 The Authors. Evolutionary Applications published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


Similar articles
-
Basis for enhanced barrier function of pigmented skin.J Invest Dermatol. 2014 Sep;134(9):2399-2407. doi: 10.1038/jid.2014.187. Epub 2014 Apr 14. J Invest Dermatol. 2014. PMID: 24732399 Free PMC article.
-
Basis for the gain and subsequent dilution of epidermal pigmentation during human evolution: The barrier and metabolic conservation hypotheses revisited.Am J Phys Anthropol. 2016 Oct;161(2):189-207. doi: 10.1002/ajpa.23030. Epub 2016 Jun 21. Am J Phys Anthropol. 2016. PMID: 27324932
-
Cutaneous side effects from laser treatment of the skin: skin cancer, scars, wounds, pigmentary changes, and purpura--use of pulsed dye laser, copper vapor laser, and argon laser.Acta Derm Venereol Suppl (Stockh). 1999;207:1-32. Acta Derm Venereol Suppl (Stockh). 1999. PMID: 10605602
-
Diversified Stimuli-Induced Inflammatory Pathways Cause Skin Pigmentation.Int J Mol Sci. 2021 Apr 12;22(8):3970. doi: 10.3390/ijms22083970. Int J Mol Sci. 2021. PMID: 33921371 Free PMC article. Review.
-
The vitamin D-folate hypothesis in human vascular health.Am J Physiol Regul Integr Comp Physiol. 2019 Sep 1;317(3):R491-R501. doi: 10.1152/ajpregu.00136.2019. Epub 2019 Jul 17. Am J Physiol Regul Integr Comp Physiol. 2019. PMID: 31314544 Free PMC article. Review.
Cited by
-
Atopic Dermatitis Across Shades of Skin.Am J Clin Dermatol. 2023 Sep;24(5):731-751. doi: 10.1007/s40257-023-00797-1. Epub 2023 Jun 19. Am J Clin Dermatol. 2023. PMID: 37336869 Review.
-
Both Prevalence and Severity of Pruritus are Associated with Age in Chinese Patients with Skin Diseases.Clin Cosmet Investig Dermatol. 2021 Mar 4;14:217-223. doi: 10.2147/CCID.S300458. eCollection 2021. Clin Cosmet Investig Dermatol. 2021. PMID: 33692631 Free PMC article.
-
Role of nitric oxide in regulating epidermal permeability barrier function.Exp Dermatol. 2022 Mar;31(3):290-298. doi: 10.1111/exd.14470. Epub 2021 Nov 1. Exp Dermatol. 2022. PMID: 34665906 Free PMC article. Review.
-
Agonism of Gpr40 Protects the Capacities of Epidermal Stem Cells (ESCs) Against Ultraviolet-B (UV-B).Drug Des Devel Ther. 2020 Nov 24;14:5143-5153. doi: 10.2147/DDDT.S252060. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33262575 Free PMC article.
-
The pigmentation phenotype of melanocytes affects their response to nitric oxide in vitro.Postepy Dermatol Alergol. 2023 Feb;40(1):150-158. doi: 10.5114/ada.2022.120130. Epub 2022 Oct 6. Postepy Dermatol Alergol. 2023. PMID: 36909911 Free PMC article.
References
-
- Abuabara, K. , You, Y. , Margolis, D. J. , Hofmann, T. J. , Risch, N. , & Jorgenson, E. (2019). Genetic ancestry does not explain increased atopic dermatitis susceptibility or worse disease control among African Americans in two large US cohorts. Journal of Allergy and Clinical Immunology. pii: S0091-6749(19)30967-4. 10.1016/j.jaci.2019.06.044. [Epub ahead of print] - DOI - PMC - PubMed
-
- Ando, H. , Niki, Y. , Ito, M. , Akiyama, K. , Matsui, M. S. , Yarosh, D. B. , & Ichihashi, M. (2012). Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. Journal of Investigative Dermatology, 132(4), 1222–1229. 10.1038/jid.2011.413 - DOI - PubMed
-
- Blome, M. W. , Cohen, A. S. , Tryon, C. A. , Brooks, A. S. , & Russell, J. (2012). The environmental context for the origins of modern human diversity: A synthesis of regional variability in African climate 150,000–30,000 years ago. Journal of Human Evolution, 62(5), 563–592. 10.1016/j.jhevol.2012.01.011 - DOI - PubMed

