microRNA-1 inhibits cardiomyocyte proliferation in mouse neonatal hearts by repressing CCND1 expression
- PMID: 31700891
- PMCID: PMC6803203
- DOI: 10.21037/atm.2019.08.68
microRNA-1 inhibits cardiomyocyte proliferation in mouse neonatal hearts by repressing CCND1 expression
Abstract
Background: The functions of microRNA-1 (miR-1) in cardiac hypertrophy, and cardiomyocyte differentiation have been investigated. However, the mechanism on how miR-1 could repress cardiomyocyte proliferation has not been fully elucidated.
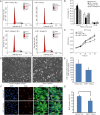
Methods: We address this issue by investigating whether miR-1 affected the proliferation of neonatal cardiomyocyte and identify some of the genes targeted by miR-1. miR-1 was over-expressed in neonatal cardiomyocytes and the effect on cell cycle and growth were analyzed by flow cytometry and Brdu-incorporation assay. Relevant vectors carrying the luciferase reporter were constructed for validation of miR-1 binding to its matching sites on the 3'-untranslated region of the predicated target mRNAs. Cardiomyocytes were co-transfected with the vectors and miR-1 mimics, then luciferase reporter assay was performed. Lastly, we examined the expression of target genes in cardiomyocytes after transfection with miR-1 mimics, as well as their normal expression pattern in 2- and 13-day-old mice hearts.
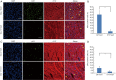
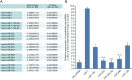
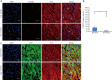
Results: We have demonstrated that miR-1 was the most significantly upregulated miRNA in 13-day-old mouse hearts compared with 2-day-old hearts. We also showed that miR-1 could repress cardiomyocyte G1/S phase transition, proliferation and viability. IGF1 and CCND1 were identified as candidate target genes regulated by miR-1. In addition, overexpression of miR-1 could suppress the expression of these two genes at the mRNA level. It could also correspondingly inhibit CCND1 expression at the protein level but not for IGF1.
Conclusions: Our results suggest that miR-1 plays an important role in inhibiting cardiomyocyte proliferation in the developing neonatal mouse heart by directly suppressing the cell-cycle regulator, CCND1.
Keywords: CCND1; cardiomyocyte proliferation; microRNA-1 (miR-1); neonatal mouse heart.
2019 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
