Neurovascular Unit as a Source of Ischemic Stroke Biomarkers-Limitations of Experimental Studies and Perspectives for Clinical Application
- PMID: 31701356
- PMCID: PMC7340668
- DOI: 10.1007/s12975-019-00744-5
Neurovascular Unit as a Source of Ischemic Stroke Biomarkers-Limitations of Experimental Studies and Perspectives for Clinical Application
Abstract
Cerebral stroke, which is one of the most frequent causes of mortality and leading cause of disability in developed countries, often leads to devastating and irreversible brain damage. Neurological and neuroradiological diagnosis of stroke, especially in its acute phase, is frequently uncertain or inconclusive. This results in difficulties in identification of patients with poor prognosis or being at high risk for complications. It also makes difficult identification of these stroke patients who could benefit from more aggressive therapies. In contrary to the cardiovascular disease, no single biomarker is available for the ischemic stroke, addressing the abovementioned issues. This justifies the need for identifying of effective diagnostic measures characterized by high specificity and sensitivity. One of the promising avenues in this area is studies on the panels of biomarkers characteristic for processes which occur in different types and phases of ischemic stroke and represent all morphological constituents of the brains' neurovascular unit (NVU). In this review, we present the current state of knowledge concerning already-used or potentially applicable biomarkers of the ischemic stroke. We also discuss the perspectives for identification of biomarkers representative for different types and phases of the ischemic stroke, as well as for different constituents of NVU, which concentration levels correlate with extent of brain damage and patients' neurological status. Finally, a critical analysis of perspectives on further improvement of the ischemic stroke diagnosis is presented.
Keywords: Astrocytes; Biomarkers; Neuroglia; Neurovascular unit; Stroke.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

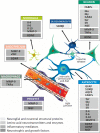
References
-
- Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. NIH Public Access; 2007 [cited 2019 Aug 27];369:293–298. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17258669. - PMC - PubMed
-
- Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. Classification of stroke subtypes. Cerebrovasc. Dis. 2009 Karger Publishers [cited 2019 Aug 27]. p. 493–501. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19342825. - PubMed
-
- Katan M, Elkind MS. The potential role of blood biomarkers in patients with ischemic stroke. Clin Transl Neurosci. 2018 SAGE PublicationsSage UK: London, England; [cited 2018 Dec 12];2:2514183X1876805. Available from: http://journals.sagepub.com/doi/10.1177/2514183X18768050 - DOI
-
- Whiteley W, Tseng MC, Sandercock P. Blood biomarkers in the diagnosis of ischemic stroke: a systematic review. Stroke. 2008 [cited 2018 Dec 8]. p. 2902–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18658039. - PubMed
-
- Atkinson AJ, Colburn WA, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001 [cited 2019 Aug 27]. p. 89–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11240971. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

