Proposed Therapeutic Range of Treosulfan in Reduced Toxicity Pediatric Allogeneic Hematopoietic Stem Cell Transplant Conditioning: Results From a Prospective Trial
- PMID: 31701524
- PMCID: PMC7484914
- DOI: 10.1002/cpt.1715
Proposed Therapeutic Range of Treosulfan in Reduced Toxicity Pediatric Allogeneic Hematopoietic Stem Cell Transplant Conditioning: Results From a Prospective Trial
Abstract
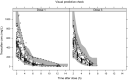
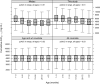
Treosulfan is given off-label in pediatric allogeneic hematopoietic stem cell transplant. This study investigated treosulfan's pharmacokinetics (PKs), efficacy, and safety in a prospective trial. Pediatric patients (n = 87) receiving treosulfan-fludarabine conditioning were followed for at least 1 year posttransplant. PKs were described with a two-compartment model. During follow-up, 11 of 87 patients died and 12 of 87 patients had low engraftment (≤ 20% myeloid chimerism). For each increase in treosulfan area under the curve from zero to infinity (AUC(0-∞) ) of 1,000 mg hour/L the hazard ratio (95% confidence interval) for mortality increase was 1.46 (1.23-1.74), and the hazard ratio for low engraftment was 0.61 (0.36-1.04). A cumulative AUC(0-∞) of 4,800 mg hour/L maximized the probability of success (> 20% engraftment and no mortality) at 82%. Probability of success with AUC(0-∞) between 80% and 125% of this target were 78% and 79%. Measuring PK at the first dose and individualizing the third dose may be required in nonmalignant disease.
© 2019 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
M.S. has received travel grants to attend meetings by Medac and honoraria for speaking engagements. A.G. has received travel grants to attend meetings by Medac. All other authors declared no competing interests for this work.
Figures


References
-
- Chiesa, R. & Veys, P . Reduced‐intensity conditioning for allogeneic stem cell transplant in primary immune deficiencies. Expert Rev. Clin. Immunol. 8, 255–266 (2012). - PubMed
-
- Bernardo, M.E. et al Allogeneic hematopoietic stem cell transplantation in thalassemia major: results of a reduced‐toxicity conditioning regimen based on the use of treosulfan. Blood 120, 473–476 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

