Wedging open a catalytic site
- PMID: 31701871
- PMCID: PMC6839915
- DOI: 10.7554/eLife.52418
Wedging open a catalytic site
Abstract
The activation mechanism of the nitric oxide receptor has been revealed by cryo-electron microscopy.
Keywords: CryoEM; allostery; biochemistry; chemical biology; cyclic GMP; manduca sexta; nitric oxide; none.
© 2019, Beuve.
Conflict of interest statement
AB No competing interests declared
Figures
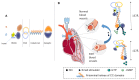
Comment on
-
Allosteric activation of the nitric oxide receptor soluble guanylate cyclase mapped by cryo-electron microscopy.Elife. 2019 Sep 30;8:e50634. doi: 10.7554/eLife.50634. Elife. 2019. PMID: 31566566 Free PMC article.
References
-
- Buys ES, Zimmer DP, Chickering J, Graul R, Chien YT, Profy A, Hadcock JR, Masferrer JL, Milne GT. Discovery and development of next generation sGC stimulators with diverse multidimensional pharmacology and broad therapeutic potential. Nitric Oxide. 2018;78:72–80. doi: 10.1016/j.niox.2018.05.009. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

