Phase II Trial of Neoadjuvant Systemic Chemotherapy Followed by Extirpative Surgery in Patients with High Grade Upper Tract Urothelial Carcinoma
- PMID: 31702432
- PMCID: PMC7735436
- DOI: 10.1097/JU.0000000000000644
Phase II Trial of Neoadjuvant Systemic Chemotherapy Followed by Extirpative Surgery in Patients with High Grade Upper Tract Urothelial Carcinoma
Abstract
Purpose: Data supporting neoadjuvant chemotherapy of high grade upper tract urothelial carcinoma are scant. In this multi-institution, prospective, phase II trial we investigated pathological complete responses after neoadjuvant chemotherapy of high grade upper tract urothelial carcinoma.
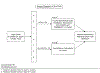
Materials and methods: Patients with high grade upper tract urothelial carcinoma in whom nephroureterectomy was planned were assigned to 4 neoadjuvant chemotherapy cycles of accelerated methotrexate, vinblastine, doxorubicin and cisplatin in those with baseline creatinine clearance greater than 50 ml per minute or gemcitabine and carboplatin in those with creatinine clearance 30 to 50 ml per minute or less. The study primary end point was a pathological complete response (ypT0N0). The accrual goal was 30 patients per arm. An 18% pathological complete response was considered worth further study while a 4% pathological complete response would not have justified pursuing this regimen. With 28 eligible patients per arm success was defined as 3 or more pathological complete responses (10.7%) in a given arm. Secondary end points included safety, renal function and oncologic outcomes.
Results: A total of 30 patients enrolled in the accelerated methotrexate, vinblastine, doxorubicin and cisplatin arm from 2015 to 2017. Six patients enrolled in the gemcitabine and carboplatin arm, which closed due to poor accrual. Of the 29 patients eligible for accelerated methotrexate, vinblastine, doxorubicin and cisplatin, including 23 men and 6 women with a median age of 65 years (range 40 to 84), 80% completed all planned treatments, 3 (10.3%) achieved ypT0N0 and 1 achieved ypT0Nx for a pathological complete response in 13.8% (90% CI 4.9-28.8). In 1 patient receiving accelerated methotrexate, vinblastine, doxorubicin and cisplatin nephroureterectomy was deferred due to grade 4 sepsis. The grade 3-4 toxicity rate was 23% in the accelerated methotrexate, vinblastine, doxorubicin and cisplatin arm with no grade 5 event.
Conclusions: Accelerated methotrexate, vinblastine, doxorubicin and cisplatin neoadjuvant chemotherapy in patients with high grade upper tract urothelial carcinoma and creatinine clearance greater than 50 ml per minute was safe and demonstrated predefined activity with a 14% pathological complete response rate. Final pathological stage ypT1 or less in more than 60% of patients is encouraging. Together the results of this prospective trial support the use of neoadjuvant chemotherapy in eligible patients with high grade upper tract urothelial carcinoma.
Keywords: carcinoma; drug therapy; nephroureterectomy; urinary tract; urothelium.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare no relevant conflicts of interest.
Figures

Comment in
-
Editorial Comment.J Urol. 2020 Apr;203(4):697. doi: 10.1097/JU.0000000000000644.01. Epub 2020 Jan 22. J Urol. 2020. PMID: 31967496 No abstract available.
-
Re: Phase II Trial of Neoadjuvant Systemic Chemotherapy Followed by Extirpative Surgery in Patients with High Grade Upper Tract Urothelial Carcinoma.Eur Urol. 2020 Jul;78(1):113-114. doi: 10.1016/j.eururo.2020.04.008. Epub 2020 May 7. Eur Urol. 2020. PMID: 32387123 No abstract available.
References
-
- Roupret M, Babjuk M, Comperat E et al.: European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update. Eur Urol, 73: 111, 2018 - PubMed
-
- Siegel RL, Miller KD, Jemal A: Cancer statistics, 2018. CA Cancer J Clin, 68: 7, 2018 - PubMed
-
- Singla N, Hutchinson R, Menegaz C et al.: Comparing Changes in Renal Function After Radical Surgery for Upper Tract Urothelial Carcinoma and Renal Cell Carcinoma. Urology, 96: 44, 2016 - PubMed
-
- Grossman HB, Natale RB, Tangen CM et al.: Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med, 349: 859, 2003 - PubMed
-
- Milowsky MI, Rumble RB, Booth CM et al.: Guideline on Muscle-Invasive and Metastatic Bladder Cancer (European Association of Urology Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol, 34: 1945, 2016 - PubMed
Publication types
MeSH terms
Grants and funding
- U10 CA180834/CA/NCI NIH HHS/United States
- UG1 CA233329/CA/NCI NIH HHS/United States
- U10 CA180802/CA/NCI NIH HHS/United States
- U10 CA180858/CA/NCI NIH HHS/United States
- UG1 CA233324/CA/NCI NIH HHS/United States
- UG1 CA233160/CA/NCI NIH HHS/United States
- UG1 CA233302/CA/NCI NIH HHS/United States
- UG1 CA233196/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- U10 CA180870/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180801/CA/NCI NIH HHS/United States
- UG1 CA233341/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

