Non-enzymatic primer extension with strand displacement
- PMID: 31702557
- PMCID: PMC6872209
- DOI: 10.7554/eLife.51888
Non-enzymatic primer extension with strand displacement
Abstract
Non-enzymatic RNA self-replication is integral to the emergence of the 'RNA World'. Despite considerable progress in non-enzymatic template copying, demonstrating a full replication cycle remains challenging due to the difficulty of separating the strands of the product duplex. Here, we report a prebiotically plausible approach to strand displacement synthesis in which short 'invader' oligonucleotides unwind an RNA duplex through a toehold/branch migration mechanism, allowing non-enzymatic primer extension on a template that was previously occupied by its complementary strand. Kinetic studies of single-step reactions suggest that following invader binding, branch migration results in a 2:3 partition of the template between open and closed states. Finally, we demonstrate continued primer extension with strand displacement by employing activated 3'-aminonucleotides, a more reactive proxy for ribonucleotides. Our study suggests that complete cycles of non-enzymatic replication of the primordial genetic material may have been facilitated by short RNA oligonucleotides.
Keywords: biochemistry; chemical biology; none; synthesis 3'-NP-DNA; synthesis RNA.
© 2019, Zhou et al.
Conflict of interest statement
LZ, SK, KH, DO, CG, TW, JS No competing interests declared
Figures












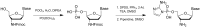

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

