Lenalidomide maintenance for diffuse large B-cell lymphoma patients responding to R-CHOP: quality of life, dosing, and safety results from the randomised controlled REMARC study
- PMID: 31702836
- PMCID: PMC7154674
- DOI: 10.1111/bjh.16300
Lenalidomide maintenance for diffuse large B-cell lymphoma patients responding to R-CHOP: quality of life, dosing, and safety results from the randomised controlled REMARC study
Abstract
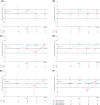
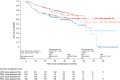
Lenalidomide maintenance therapy prolonged progression-free survival (PFS) versus placebo in elderly patients with diffuse large B-cell lymphoma (DLBCL) responding to induction chemotherapy in the phase 3 REMARC study. This subpopulation analysis assessed the impact of lenalidomide maintenance and treatment-emergent adverse events (TEAEs) on health-related quality of life (HRQOL). Global health status (GHS), and physical functioning and fatigue subscales were evaluated in patients who completed the European Organisation for Research and Treatment of Cancer quality-of-life questionnaire-C30 v3.0. The impact of TEAEs classified post hoc as subjective (patients can feel) or observable (only measurable by physicians) on dose reductions and discontinuations was assessed. Among 457 patients (lenalidomide, n = 229; placebo, n = 228), mean (standard deviation) GHS was similar between treatment arms [68·2 (20·7) Versus 72·0 (17·8)] at randomisation and remained similar during maintenance. Patients receiving lenalidomide experienced no meaningful changes in GHS, physical functioning, or fatigue. Observable TEAEs were more common (81·1% Versus 66·3%) and more likely to lead to dose reductions, than subjective TEAEs in both arms. PFS was superior in the lenalidomide arm regardless of dose reduction. Lenalidomide maintenance prolonged PFS and did not negatively impact HRQOL in patients with DLBCL despite TEAEs being more common, when compared with placebo.
Keywords: non-Hodgkin lymphoma; quality of life; therapy.
© 2019 The Authors. British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
CT has been on an advisory board for Celgene Corporation, Gilead, Kyte, Novartis, and Roche. SH is an employee of Celgene Corporation. R‐OC has received grants, personal fees, and non‐financial support from Roche, Takeda, and Gilead; and personal fees from Bristol‐Myers Squibb, Merck, and Janssen. NM has no conflict of interest to disclose. AP has no conflict of interest to disclose. FM has been on an advisory board and provided scientific lectures to Celgene Corporation and Roche; has been on an advisory board for Gilead and Bristol‐Myers Squibb; provided scientific lectures for Janssen; and provided consultancy to Epizyme. CF has no conflict of interest to disclose. ND has no conflict of interest to disclose. KVE has no conflict of interest to disclose. LO has no conflict of interest to disclose. RB has no conflict of interest to disclose. GMP has no conflict of interest to disclose. EN‐V has no conflict of interest to disclose. JA has no conflict of interest to disclose. OF has no conflict of interest to disclose. SS has no conflict of interest to disclose. J‐CE has no conflict of interest to disclose. PL‐H has no conflict of interest to disclose. DB has received research funding. ST has no conflict of interest to disclose. DD has no conflict of interest to disclose. HG has no conflict of interest to disclose. RC has no conflict of interest to disclose. KLD has no conflict of interest to disclose. MGdS has received a research grant from, and provided consultancy to, Gilead Sciences; been on an advisory board for AbbVie; received institutional payments and travel reimbursement from Roche; provided consultancy to, and received travel reimbursement from, Janssen Cilag; and received travel reimbursement from Celgene Corporation. SG has no conflict of interest to disclose. JT has received non‐financial support from Celgene Corporation; and received clinical trials funding from BeiGene, Celgene, Janssen, PCYC, and Roche. JC has received travel reimbursement from Amgen and Celgene Corporation. DC has no conflict of interest to disclose. RG has received honoraria, research funding, travel and accommodation expenses, and provided consultancy or been on an advisory board for, Celgene Corporation, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol‐Myers Squibb, MSD, Sandoz, AbbVie, and Janssen. AMC has no conflict of interest to disclose. PG has received a grant and personal fees from Takeda. LR has no conflict of interest to disclose. KT is a former employee of, and has stock ownership in, Celgene Corporation. MLC is an employee of Celgene Corporation. HT has received personal fees and non‐financial support from Roche; and received personal fees from AstraZeneca, Bristol‐Myers Squibb, and Karyopharm.
Figures
References
-
- Aaronson, N.K. , Ahmedzai, S. , Bergman, B. , Bullinger, M. , Cull, A. , Duez, N.J. , Filiberti, A. , Flechtner, H. , Fleishman, S.B. , de Haes, J.C. , Kaasa, S. , Klee, M. , Osoba, D. , Razavi, D. , Rofe, P.B. , Schraub, S. , Sneeuw, K. , Sullivan, M. & Takeda, F. (1993) The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute, 85, 365–376. - PubMed
-
- Attal, M. , Lauwers‐Cances, V. , Marit, G. , Caillot, D. , Moreau, P. , Facon, T. , Stoppa, A.M. , Hulin, C. , Benboubker, L. , Garderet, L. , Decaux, O. , Leyvraz, O. , Vekemans, M.C. , Voillat, L. , Michallet, M. , Pegourie, B. , Dumontet, C. , Roussel, M. , Leleu, X. , Mathiot, C. , Payen, C. , Avet‐Loiseau, H. , Harousseau, J.L. & for the IFM Investigators . (2012) Lenalidomide maintenance after stem‐cell transplantation for multiple myeloma. New England Journal of Medicine, 366, 1782–1791. - PubMed
-
- Basch, E. , Reeve, B.B. , Mitchell, S.A. , Clauser, S.B. , Minasian, L.M. , Dueck, A.C. , Mendoza, T.R. , Hay, J. , Atkinson, T.M. , Abernethy, A.P. , Bruner, D.W. , Cleeland, C.S. , Sloan, J.A. , Chilukuri, R. , Baumgartner, P. , Denicoff, A. , St Germain, D. , O'Mara, A.M. , Chen, A. , Kelaghan, J. , Bennett, A.V. , Sit, L. , Rogak, L. , Barz, A. , Paul, D.B. & Schrag, D. (2014) Development of the National Cancer Institute's patient‐reported outcomes version of the common terminology criteria for adverse events (PRO‐CTCAE). Journal of the National Cancer Institute, 106, dju244. - PMC - PubMed
-
- Bernhard, J. , Cella, D.F. , Coates, A.S. , Fallowfield, L. , Ganz, P.A. , Moinpour, C.M. , Mosconi, P. , Osoba, D. , Simes, J. & Hürny, C. (1998) Missing quality of life data in cancer clinical trials: serious problems and challenges. Statistics in Medicine, 17, 517–532. - PubMed
-
- Chiappella, A. , Castellino, A. , Nicolosi, M. , Santambrogio, E. & Vitolo, U. (2017a) Diffuse large B‐cell lymphoma in the elderly: standard treatment and new perspectives. Expert Review of Hematology, 10, 289–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

