NOP53 as A Candidate Modifier Locus for Familial Non-Medullary Thyroid Cancer
- PMID: 31703244
- PMCID: PMC6896177
- DOI: 10.3390/genes10110899
NOP53 as A Candidate Modifier Locus for Familial Non-Medullary Thyroid Cancer
Abstract
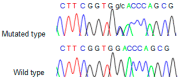
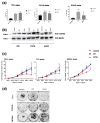
Nonsyndromic familial non-medullary thyroid cancer (FNMTC) represents 3-9% of thyroid cancers, but the susceptibility gene(s) remain unknown. We designed this multicenter study to analyze families with nonsyndromic FNMTC and identify candidate susceptibility genes. We performed exome sequencing of DNA from four affected individuals from one kindred, with five cases of nonsyndromic FNMTC. Single Nucleotide Variants, and insertions and deletions that segregated with all the affected members, were analyzed by Sanger sequencing in 44 additional families with FNMTC (37 with two affected members, and seven with three or more affected members), as well as in an independent control group of 100 subjects. We identified the germline variant p. Asp31His in NOP53 gene (rs78530808, MAF 1.8%) present in all affected members in three families with nonsyndromic FNMTC, and not present in unaffected spouses. Our functional studies of NOP53 in thyroid cancer cell lines showed an oncogenic function. Immunohistochemistry exhibited increased NOP53 protein expression in tumor samples from affected family members, compared with normal adjacent thyroid tissue. Given the relatively high frequency of the variant in the general population, these findings suggest that instead of a causative gene, NOP53 is likely a low-penetrant gene implicated in FNMTC, possibly a modifier.
Keywords: NOP53; genetic abnormalities; molecular testing; oncogenic mutations; thyroid cancer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Sahasrabudhe R., Stultz J., Williamson J., Lott P., Estrada A., Bohorquez M., Palles C., Polanco-Echeverry G., Jaeger E., Martin L., et al. The HABP2 G534E variant is an unlikely cause of familial non-medullary thyroid cancer. J. Clin. Endocrinol. Metab. 2016;10:1098–1103. doi: 10.1210/jc.2015-3928. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

