Evolution of H5-Type Avian Influenza A Virus Towards Mammalian Tropism in Egypt, 2014 to 2015
- PMID: 31703251
- PMCID: PMC6963730
- DOI: 10.3390/pathogens8040224
Evolution of H5-Type Avian Influenza A Virus Towards Mammalian Tropism in Egypt, 2014 to 2015
Abstract
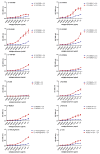
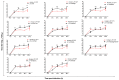
Highly pathogenic avian influenza viruses (HPAIV) of the H5-subtype have circulated continuously in Egypt since 2006, resulting in numerous poultry outbreaks and considerable sporadic human infections. The extensive circulation and wide spread of these viruses in domestic poultry have resulted in various evolutionary changes with a dramatic impact on viral transmission ability to contact mammals including humans. The transmitted viruses are either (1) adapted well enough in their avian hosts to readily infect mammals, or (2) adapted in the new mammalian hosts to improve their fitness. In both cases, avian influenza viruses (AIVs) acquire various host-specific adaptations. These adaptive variations are not all well-known or thoroughly characterized. In this study, a phylogenetic algorithm based on the informational spectrum method, designated hereafter as ISM, was applied to analyze the affinity of H5-type HA proteins of Egyptian AIV isolates (2006-2015) towards human-type cell receptors. To characterize AIV H5-HA proteins displaying high ISM values reflecting an increased tendency of the HA towards human-type receptors, recombinant IV expressing monobasic, low pathogenic (LP) H5-HA versions in the background of the human influenza virus A/PR/8/1934(H1N1) (LP 7+1), were generated. These viruses were compared with a LP 7+1 expressing a monobasic H5-HA from a human origin virus isolate (human LP-7271), for their receptor binding specificity (ISM), in vitro replication efficiency and in vivo pathogenicity in mammals. Interestingly, using ISM analysis, we identified a LP 7+1 virus (LP-S10739C) expressing the monobasic H5-HA of AIV A/Chicken/Egypt/S10739C/2015(H5N1) that showed high affinity towards human-type receptors. This in silico prediction was reflected by a higher in vitro replication efficiency in mammalian cell cultures and a higher virulence in mice as compared with LP-7271. Sequence comparison between the LP-S10739C and the LP-7271 H5-HA, revealed distinct amino acid changes. Their contribution to the increased mammalian receptor propensity of LP-S10739C demands further investigation to better deduce the molecular determinant behind the reported high morbidity of 2014 to 2015 HPAI H5N1 virus in humans in Egypt. This study provides insights into the evolution of Egyptian H5 HPAIVs and highlights the need to identify the viral evolution in order to recognize emerging AIV with the potential to threaten human and animal populations.
Keywords: ISM analysis; evolution; human-type receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

