Design, Synthesis and Biological Evaluation of Diosgenin-Amino Acid Derivatives with Dual Functions of Neuroprotection and Angiogenesis
- PMID: 31703284
- PMCID: PMC6891328
- DOI: 10.3390/molecules24224025
Design, Synthesis and Biological Evaluation of Diosgenin-Amino Acid Derivatives with Dual Functions of Neuroprotection and Angiogenesis
Abstract
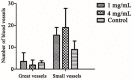
Diosgenin, a natural product with steroidal structure, has a wide range of clinical applications in China. It also shows great potential in the treatment of blood clots and nerve damage. To enhance the bioavailability as well as efficacy of diosgenin, eighteen diosgenin-amino acid derivatives were designed and synthesized. The neuroprotective effects of these compounds were evaluated by SH-SY5Y cell line and the biosafety was evaluated by H9c2 cell line. The results displayed that part of the derivatives' activities (EC50 < 20 μM) were higher than positive control edaravone (EC50 = 21.60 ± 3.04 μM), among which, DG-15 (EC50 = 6.86 ± 0.69 μM) exhibited the best neuroprotection. Meanwhile, biosafety evaluation showed that DG-15 had no cytotoxicity on H9c2 cell lines. Interestingly, combined neuroprotective and cytotoxic results, part of the derivatives without their protecting group were superior to compounds with protecting group. Subsequently, Giemsa staining and DAPI (4',6-diamidino-2-phenylindole) staining indicated that DG-15 had a protective effect on damaged SH-SY5Y cells by reducing apoptosis. Moreover, DG-15 showed a higher role in promoting angiogenesis at high concentrations (4 mg/mL) on the chorioallantoic membrane model. This finding displayed that DG-15 had dual functions of neuroprotection and angiogenesis, which provided further insight into designing agent for the application in treatment of ischemic stroke.
Keywords: CAM model; angiogenesis; diosgenin-amino acids derivatives; neuroprotection.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
- 81603256/National Natural Science Foundation of China
- CACM-2018-QNRC2-B08/project of China Association of Chinese Medicine
- BUCM-2019-JCRC002, BUCM-2018-2020 and 2019-JYB-TD005/Fundamental Research Funds for the Central Universities
- Beijing, 100102/Beijing Key Laboratory for Basic and Development Research on Chinese Medicine
LinkOut - more resources
Full Text Sources

