Network Meta-Analysis of Efficacy and Safety of Chemotherapy and Target Therapy in the First-Line Setting of Advanced Pancreatic Cancer
- PMID: 31703359
- PMCID: PMC6895788
- DOI: 10.3390/cancers11111746
Network Meta-Analysis of Efficacy and Safety of Chemotherapy and Target Therapy in the First-Line Setting of Advanced Pancreatic Cancer
Abstract
Both gemcitabine and fluoropyrimidine are recommended backbones in the first-line treatment of pancreatic ductal adenocarcinoma (PDAC). To compare the efficacy and safety of these two therapeutic backbones, and to investigate the optimal therapies, we conducted a network meta-analysis. By retrospective analysis of randomized controlled trials (RCT), the most preferred therapeutic regimen may be predicted. The eligible RCTs of the gemcitabine-based therapies and fluoropyrimidine-based therapies were searched up to 31 August 2019. In a frequentist network meta-analysis, treatments were compared and ranked according to overall survival (OS) and progression-free survival (PFS). Thirty-two trials with 10,729 patients were included. The network meta-analyses results for overall survival and progression-free survival showed that fluoropyrimidine-based therapy seems to be the most effective treatment choice. Compared to gemcitabine combined with taxanes or immunotherapy, fluoropyrimidine-based therapy had comparable treatment effects (PFS: 0.67, p-Value = 0.11; 0.76, p-Value = 0.32; OS: 0.80, p-Value = 0.16; 0.77, p-Value = 0.21). Moreover, the combination of immunotherapy and gemcitabine had tolerable toxicities. Based on current evidence, fluoropyrimidine-based therapies and the combination of gemcitabine and taxanes were the most effective therapies in the advanced pancreatic cancer, and the combination of immunotherapy and gemcitabine can be developed into a new form of therapy.
Keywords: advanced pancreatic cancer; adverse effects; chemotherapy; efficacy outcomes; target therapy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results of the study.
Figures
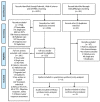
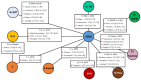
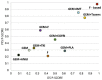
References
-
- Sohal D.P.S., Kennedy E.B., Khorana A., Copur M.S., Crane C.H., Garrido-Laguna I., Krishnamurthi S., Moravek C., O’Reilly E.M., Philip P.A., et al. Metastatic Pancreatic Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018;36:2545–2556. doi: 10.1200/JCO.2018.78.9636. - DOI - PMC - PubMed
-
- Ducreux M., Cuhna A.S., Caramella C., Hollebecque A., Burtin P., Goere D., Seufferlein T., Haustermans K., Van Laethem J.L., Conroy T., et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015;26:v56–v68. doi: 10.1093/annonc/mdv295. - DOI - PubMed
-
- Moore M.J., Goldstein D., Hamm J., Figer A., Hecht J.R., Gallinger S., Au H.J., Murawa P., Walde D., Wolff R.A., et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

