Determination of Picomolar Concentrations of Paraoxon in Human Urine by Fluorescence-Based Enzymatic Assay
- PMID: 31703397
- PMCID: PMC6891394
- DOI: 10.3390/s19224852
Determination of Picomolar Concentrations of Paraoxon in Human Urine by Fluorescence-Based Enzymatic Assay
Abstract
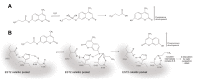
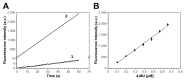
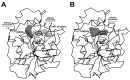
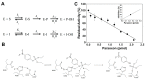
Organophosphate (OP) pesticides are widely used in the agricultural field and in the prevention of pest infestation in private and public areas of cities. Despite their unquestionable utility, several of these compounds demonstrate toxic effects to the environment and human health. In particular, the occurrence of some organophosphate pesticides is correlated to the incidence of nervous system disorders, especially in children. The detection of pesticide residues in the human body represents an important task to preserve human health. In our work we propose the use of esterase-based biosensors as a viable alternative to the expensive and time-consuming systems currently used for their detection in human fluids. Using the esterase-2 activity, coupled with a fluorescence inhibition assay, we are able to detect very low concentration levels of diethyl (4-nitrophenyl) phosphate (paraoxon) in the range of the femtomole (fmol). Method robustness tests indicate the stability of esterase-2 in a diluted solution of 4% human urine, and we are able to accurately determine concentration levels of paraoxon in the range from 0.1 to 2 picomoles (pmol). The system sensitivity for OP detection is calculated at 524 ± 14.15 fmol of paraoxon recognized at 10% of inhibition, with an estimated limit of quantification of 262 ± 8.12 pmol mL-1. These values are comparable with the most recent analysis methods based on mass spectrometry carried out on human samples for pesticide detection. This research represents a starting point to develop cheap and fast testing methods for a rapid screening of toxic substances in human samples.
Keywords: 4-methylumbelliferyl butyrate; Alicyclobacillus acidocaldarius; EST2; Organophosphate pesticides; biomonitoring; biosensor; esterase-2; fluorescence; human urine; paraoxon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

