Conjugation of Amisulpride, an Anti-Psychotic Agent, with 5-Aminosalicylic Acid via an Azo Bond Yields an Orally Active Mutual Prodrug against Rat Colitis
- PMID: 31703411
- PMCID: PMC6920822
- DOI: 10.3390/pharmaceutics11110585
Conjugation of Amisulpride, an Anti-Psychotic Agent, with 5-Aminosalicylic Acid via an Azo Bond Yields an Orally Active Mutual Prodrug against Rat Colitis
Abstract
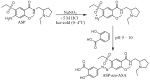
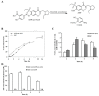
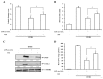
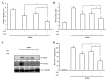
Amisulpride (ASP), an anti-psychotic agent, is a pharmacologically equivalent to sulpiride (SP). Because SP demonstrates anti-ulcer and anti-colitic activities, ASP with an aniline moiety was azo-coupled to salicylic acid to generate 5-(aminoethanoylsulfamoyl)-N-[(1-ethylpyrrolidin-2-yl)methyl]-2-methoxybenzamide (ASP-azo-ASA), with the expectation that it would act as a colon-specific mutual prodrug against colitis. Following a 24 h incubation, approximately 80% of ASP-azo-ASA was cleaved to form ASP and 5-aminosalicylic acid (5-ASA) in the cecal contents, whereas it remained stable in the small intestinal contents. Oral gavage of ASP-azo-ASA (oral ASP-azo-ASA) delivered 5-ASA to the cecum to levels comparable with those observed for sulfasalazine (SSZ; clinical colon-specific prodrug of 5-ASA) and without detectable concentrations of ASP in the blood, indicating efficient colonic delivery. Oral ASP-azo-ASA ameliorated 2, 4-dinitrobenzenesulfonic acid hydrate (DNBS)-induced colitis in rats more effectively than oral SSZ. Additionally, oral ASP-azo-ASA lowered the levels of inflammatory mediators in the inflamed distal colon more effectively than oral SSZ. Combined treatment with 5-ASA and ASP via the rectal route more effectively reversed colonic damage and inflammation than treatment with 5-ASA or ASP alone, confirming the mutual anti-colitic actions of 5-ASA and ASP. In conclusion, ASP-azo-ASA is an orally active mutual prodrug against rat colitis with limited systemic absorption of ASP.
Keywords: 5-Aminosalicylic acid; amisulpride; colon-specific drug delivery; inflammatory bowel disease; mutual prodru.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A colon-specific prodrug of metoclopramide ameliorates colitis in an experimental rat model.Drug Des Devel Ther. 2018 Dec 28;13:231-242. doi: 10.2147/DDDT.S185257. eCollection 2019. Drug Des Devel Ther. 2018. PMID: 30643389 Free PMC article.
-
Therapeutic switching of sulpiride, an anti-psychotic and prokinetic drug, to an anti-colitic drug using colon-specific drug delivery.Drug Deliv Transl Res. 2019 Feb;9(1):334-343. doi: 10.1007/s13346-018-00599-7. Drug Deliv Transl Res. 2019. PMID: 30426357
-
5-Aminosalicylic Acid Azo-Linked to Procainamide Acts as an Anticolitic Mutual Prodrug via Additive Inhibition of Nuclear Factor kappaB.Mol Pharm. 2016 Jun 6;13(6):2126-35. doi: 10.1021/acs.molpharmaceut.6b00294. Epub 2016 May 5. Mol Pharm. 2016. PMID: 27112518
-
Clinical pharmacokinetics of sulphasalazine, its metabolites and other prodrugs of 5-aminosalicylic acid.Clin Pharmacokinet. 1985 Jul-Aug;10(4):285-302. doi: 10.2165/00003088-198510040-00001. Clin Pharmacokinet. 1985. PMID: 2864155 Review.
-
5-Aminosalicylic acid containing drugs. Delivery, fate, and possible clinical implications in man.Dan Med Bull. 2000 Feb;47(1):20-41. Dan Med Bull. 2000. PMID: 10709142 Review.
Cited by
-
Analysis of Gut Microbiota in Patients with Exacerbated Symptoms of Schizophrenia following Therapy with Amisulpride: A Pilot Study.Behav Neurol. 2022 Mar 5;2022:4262094. doi: 10.1155/2022/4262094. eCollection 2022. Behav Neurol. 2022. PMID: 35287288 Free PMC article.
-
GelNB molecular coating as a biophysical barrier to isolate intestinal irritating metabolites and regulate intestinal microbial homeostasis in the treatment of inflammatory bowel disease.Bioact Mater. 2022 Apr 21;19:251-267. doi: 10.1016/j.bioactmat.2022.04.001. eCollection 2023 Jan. Bioact Mater. 2022. PMID: 35510173 Free PMC article.
-
Dopamine, Immunity, and Disease.Pharmacol Rev. 2023 Jan;75(1):62-158. doi: 10.1124/pharmrev.122.000618. Epub 2022 Dec 8. Pharmacol Rev. 2023. PMID: 36757901 Free PMC article. Review.
-
Disulfide based prodrugs for cancer therapy.RSC Adv. 2020 Jun 25;10(41):24397-24409. doi: 10.1039/d0ra04155f. eCollection 2020 Jun 24. RSC Adv. 2020. PMID: 35516223 Free PMC article. Review.
-
The influence of the gut microbiota on the bioavailability of oral drugs.Acta Pharm Sin B. 2021 Jul;11(7):1789-1812. doi: 10.1016/j.apsb.2020.09.013. Epub 2020 Sep 28. Acta Pharm Sin B. 2021. PMID: 34386321 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

