Domatinostat favors the immunotherapy response by modulating the tumor immune microenvironment (TIME)
- PMID: 31703604
- PMCID: PMC6839078
- DOI: 10.1186/s40425-019-0745-3
Domatinostat favors the immunotherapy response by modulating the tumor immune microenvironment (TIME)
Abstract
Background: The efficacy of PD-(L)1 blockade depends on the composition of the tumor immune microenvironment (TIME) and is generally higher in tumors with pre-existing cytotoxic T cells (CTL) than in those with low CTL numbers. Nonetheless, a significant proportion of patients with pre-existing immunity fail to respond, indicating a therapeutic potential for combining PD-(L)1 blockade with additional immunomodulatory agents in both CTL-high and -low immune phenotypes. Here, we evaluated domatinostat (4SC-202), a class I-selective histone deacetylase (HDAC) inhibitor, for its effect on the TIME and its antitumoral efficacy using syngeneic mouse models with CTL-high or CTL-low tumors.
Methods: Domatinostat was evaluated in PD-1 blockade-insensitive CTL-low (CT26) and CTL-high (C38) syngeneic models alone and in combination with different immune-inhibitory and -stimulatory approaches. Effects on the immunophenotype were assessed via flow cytometry and RNA-seq analyses. The changes in RNA-seq-based immune signatures determined in a murine setting were investigated in patient samples from the first-dose cohort of the SENSITIZE trial (NCT03278665) evaluating domatinostat combined with pembrolizumab in advanced-stage melanoma patients refractory/nonresponding to PD-1 blockade.
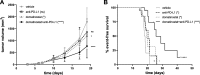
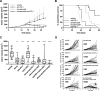
Results: Domatinostat increased the expression of antigen-presenting machinery (APM) genes and MHC class I and II molecules, along with CTL infiltration, in tumors of both immune phenotypes. In combination with PD-(L)1 blockade, domatinostat augmented antitumor effects substantially above the effects of single-agent therapies, displaying greater benefit in tumors with pre-existing CTLs. In this setting, the combination of domatinostat with agonistic anti-4-1BB or both PD-1 and LAG3 blockade further increased the antitumor efficacy. In CTL-low tumors, domatinostat enhanced the expression of genes known to reinforce immune responses against tumors. Specifically, domatinostat increased the expression of Ifng and genes associated with responses to pembrolizumab and nivolumab. Clinically, these findings were confirmed in patients with advanced melanoma treated with domatinostat for 14 days, who demonstrated elevated expression of APM and MHC genes, the IFNG gene, and the IFN-γ and pembrolizumab response signatures in individual tumor samples.
Conclusion: In summary, these data suggest a promising potential of domatinostat in combination with immunotherapy to improve the outcome of refractory cancer patients.
Keywords: Checkpoint inhibitor; Domatinostat; HDAC; IFN-γ signature; Immunotherapy; PD-1 blockade response signature; Tumor immune microenvironment.
Conflict of interest statement
ACB, UP, KK, TD, RB, FH, AA, TW, SH are employees of 4SC; TD, SH are stakeholders of 4SC. FH, RB, and SH are currently applying for patents covering combination of domatinostat with immunotherapies.
Figures





References
-
- Hegde PS, Karanikas V, Evers S. The where, the when, and the how of immune monitoring for Cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res. 2016;22:1865–1874. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
