Hydrogen sulfide attenuates cigarette smoke-induced airway remodeling by upregulating SIRT1 signaling pathway
- PMID: 31704583
- PMCID: PMC6854091
- DOI: 10.1016/j.redox.2019.101356
Hydrogen sulfide attenuates cigarette smoke-induced airway remodeling by upregulating SIRT1 signaling pathway
Abstract
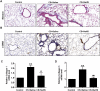
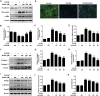
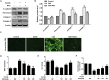
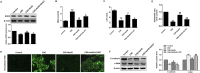
Airway remodeling is one of the characteristics for chronic obstructive pulmonary disease (COPD). The mechanism underlying airway remodeling is associated with epithelial-mesenchymal transition (EMT) in the small airways of smokers and patients with COPD. Sirtuin 1 (SIRT1) is able to reduce oxidative stress, and to modulate EMT. Here, we investigated the effects and mechanisms of hydrogen sulfide (H2S) on pulmonary EMT in vitro and in vivo. We found that H2S donor NaHS inhibited cigarette smoke (CS)-induced airway remodeling, EMT and collagen deposition in mouse lungs. In human bronchial epithelial 16HBE cells, NaHS treatment also reduced CS extract (CSE)-induced EMT, collagen deposition and oxidative stress. Mechanistically, NaHS upregulated SIRT1 expression, but inhibited activation of TGF-β1/Smad3 signaling in vivo and in vitro. SIRT1 inhibition by a specific inhibitor EX527 significantly attenuated or abolished the ability of NaHS to reverse the CSE-induced oxidative stress. SIRT1 inhibition also abolished the protection of NaHS against CSE-induced EMT. Moreover, SIRT1 activation attenuated CSE-induced EMT by modifying TGF-β1-mediated Smad3 transactivation. In conclusion, H2S prevented CS-induced airway remodeling in mice by reversing oxidative stress and EMT, which was partially ameliorated by SIRT1 activation. These findings suggest that H2S may have therapeutic potential for the prevention and treatment of COPD.
Keywords: Airway remodeling; COPD; Epithelial-mesenchymal transition; Hydrogen sulfide; Oxidative stress; Sirtuin 1.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors have declared that there is no conflict of interest.
Figures



Similar articles
-
Hydrogen sulfide attenuates mitochondrial dysfunction-induced cellular senescence and apoptosis in alveolar epithelial cells by upregulating sirtuin 1.Aging (Albany NY). 2019 Dec 23;11(24):11844-11864. doi: 10.18632/aging.102454. Epub 2019 Dec 23. Aging (Albany NY). 2019. PMID: 31881011 Free PMC article.
-
Role of Digoxin in Preventing Cigarette Smoke-Induced COPD via HIF-1α Inhibition in a Mouse Model.Int J Chron Obstruct Pulmon Dis. 2025 May 23;20:1665-1678. doi: 10.2147/COPD.S493856. eCollection 2025. Int J Chron Obstruct Pulmon Dis. 2025. PMID: 40433398 Free PMC article.
-
Mechanism of cigarette smoke in promoting small airway remodeling in mice via STAT 3 / PINK 1-Parkin / EMT.Free Radic Biol Med. 2024 Nov 1;224:447-456. doi: 10.1016/j.freeradbiomed.2024.08.036. Epub 2024 Aug 29. Free Radic Biol Med. 2024. PMID: 39214258
-
The effects of epithelial-mesenchymal transitions in COPD induced by cigarette smoke: an update.Respir Res. 2022 Aug 31;23(1):225. doi: 10.1186/s12931-022-02153-z. Respir Res. 2022. PMID: 36045410 Free PMC article. Review.
-
Narrower insight to SIRT1 role in cancer: A potential therapeutic target to control epithelial-mesenchymal transition in cancer cells.J Cell Physiol. 2018 Jun;233(6):4443-4457. doi: 10.1002/jcp.26302. Epub 2018 Jan 15. J Cell Physiol. 2018. PMID: 29194618 Review.
Cited by
-
Hydrogen Sulfide Promotes Cardiomyocyte Proliferation and Heart Regeneration via ROS Scavenging.Oxid Med Cell Longev. 2020 May 21;2020:1412696. doi: 10.1155/2020/1412696. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32566074 Free PMC article.
-
The Novel Regulatory Role of the lncRNA-miRNA-mRNA Axis in Chronic Inflammatory Airway Diseases.Front Mol Biosci. 2022 Jun 13;9:927549. doi: 10.3389/fmolb.2022.927549. eCollection 2022. Front Mol Biosci. 2022. PMID: 35769905 Free PMC article. Review.
-
Hydrogen sulfide enhances adult neurogenesis in a mouse model of Parkinson's disease.Neural Regen Res. 2021 Jul;16(7):1353-1358. doi: 10.4103/1673-5374.301026. Neural Regen Res. 2021. PMID: 33318417 Free PMC article.
-
Defective protein persulfidation is involved in obesity associated skeletal muscle dysfunction: role of SIRT-1.Redox Biol. 2025 Jun;83:103645. doi: 10.1016/j.redox.2025.103645. Epub 2025 Apr 22. Redox Biol. 2025. PMID: 40318302 Free PMC article.
-
MiR-23a-5p alleviates chronic obstructive pulmonary disease through targeted regulation of RAGE-ROS pathway.Respir Res. 2024 Feb 20;25(1):93. doi: 10.1186/s12931-024-02736-y. Respir Res. 2024. PMID: 38378600 Free PMC article.
References
-
- Szilasi M., Dolinay T., Nemes Z., Strausz J. Pathology of chronic obstructive pulmonary disease. Pathol. Oncol. Res. 2006;12(1):52–60. - PubMed
-
- Stewart J.I., Criner G.J. The small airways in chronic obstructive pulmonary disease: pathology and effects on disease progression and survival. Curr. Opin. Pulm. Med. 2013;19(2):109–115. - PubMed
-
- Wang Y., Jia M., Yan X., Cao L., Barnes P.J., Adcock I.M., Huang M., Yao X. Increased neutrophil gelatinase-associated lipocalin (NGAL) promotes airway remodelling in chronic obstructive pulmonary disease. Clin. Sci. (Lond.) 2017;131(11):1147–1159. - PubMed
-
- Hirota N., Martin J.G. Mechanisms of airway remodeling. Chest. 2013;144(3):1026–1032. - PubMed
-
- Milara J., Peiro T., Serrano A., Cortijo J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax. 2013;68(5):410–420. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

