Medium-Chain Fatty Acid Synthesis by " Candidatus Weimeria bifida" gen. nov., sp. nov., and " Candidatus Pseudoramibacter fermentans" sp. nov
- PMID: 31704684
- PMCID: PMC6974650
- DOI: 10.1128/AEM.02242-19
Medium-Chain Fatty Acid Synthesis by " Candidatus Weimeria bifida" gen. nov., sp. nov., and " Candidatus Pseudoramibacter fermentans" sp. nov
Abstract
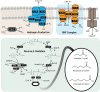
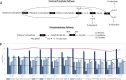
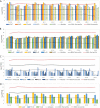
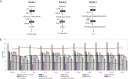
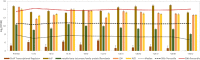
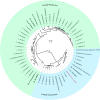
Chain elongation is emerging as a bioprocess to produce valuable medium-chain fatty acids (MCFA; 6 to 8 carbons in length) from organic waste streams by harnessing the metabolism of anaerobic microbiomes. Although our understanding of chain elongation physiology is still evolving, the reverse β-oxidation pathway has been identified as a key metabolic function to elongate the intermediate products of fermentation to MCFA. Here, we describe two uncultured chain-elongating microorganisms that were enriched in an anaerobic microbiome transforming the residues from a lignocellulosic biorefining process. Based on a multi-omic analysis, we describe "Candidatus Weimeria bifida" gen. nov., sp. nov., and "Candidatus Pseudoramibacter fermentans" sp. nov., both predicted to produce MCFA but using different substrates. The analysis of a time series metatranscriptomic data set suggests that "Ca Weimeria bifida" is an effective xylose utilizer since both the pentose phosphate pathway and the bifid shunt are active. Furthermore, the metatranscriptomic data suggest that energy conservation during MCFA production in this organism is essential and occurs via the creation of an ion motive force using both the RNF complex and an energy-conserving hydrogenase. For "Ca Pseudoramibacter fermentans," predicted to produce MCFA from lactate, the metatranscriptomic analysis reveals the activity of an electron-confurcating lactate dehydrogenase, energy conservation via the RNF complex, H2 production for redox balance, and glycerol utilization. A thermodynamic analysis also suggests the possibility of glycerol being a substrate for MCFA production by "Ca Pseudoramibacter fermentans." In total, this work reveals unknown characteristics of MCFA production in two novel organisms.IMPORTANCE Chain elongation by medium-chain fatty acid (MCFA)-producing microbiomes offers an opportunity to produce valuable chemicals from organic streams that would otherwise be considered waste. However, the physiology and energetics of chain elongation are only beginning to be studied, and many of these organisms remain uncultured. We analyzed MCFA production by two uncultured organisms that were identified as the main MCFA producers in a microbial community enriched from an anaerobic digester; this characterization, which is based on meta-multi-omic analysis, complements the knowledge that has been acquired from pure-culture studies. The analysis revealed previously unreported features of the metabolism of MCFA-producing organisms.
Keywords: Ech complex; Firmicutes; MCFA; Pseudoramibacter; RNF complex; hydrogenase; “Ca. Weimeria,” chain elongation.
Copyright © 2020 Scarborough et al.
Figures





References
-
- Angenent LT, Richter H, Buckel W, Spirito CM, Steinbusch KJJ, Plugge CM, Strik D, Grootscholten TIM, Buisman CJN, Hamelers H. 2016. Chain elongation with reactor microbiomes: open-culture biotechnology to produce biochemicals. Environ Sci Technol 50:2796–2810. doi:10.1021/acs.est.5b04847. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

