Multicenter Randomized Controlled Trial of Vitamin K Antagonist Replacement by Rivaroxaban with or without Vitamin K2 in Hemodialysis Patients with Atrial Fibrillation: the Valkyrie Study
- PMID: 31704740
- PMCID: PMC6935010
- DOI: 10.1681/ASN.2019060579
Multicenter Randomized Controlled Trial of Vitamin K Antagonist Replacement by Rivaroxaban with or without Vitamin K2 in Hemodialysis Patients with Atrial Fibrillation: the Valkyrie Study
Abstract
Background: Vitamin K antagonists (VKAs), although commonly used to reduce thromboembolic risk in atrial fibrillation, have been incriminated as probable cause of accelerated vascular calcification (VC) in patients on hemodialysis. Functional vitamin K deficiency may further contribute to their susceptibility for VC. We investigated the effect of vitamin K status on VC progression in 132 patients on hemodialysis with atrial fibrillation treated with VKAs or qualifying for anticoagulation.
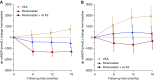
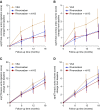
Methods: Patients were randomized to VKAs with target INR 2-3, rivaroxaban 10 mg daily, or rivaroxaban 10 mg daily plus vitamin K2 2000 µg thrice weekly during 18 months. Systemic dp-ucMGP levels were quantified to assess vascular vitamin K status. Cardiac and thoracic aorta calcium scores and pulse wave velocity were measured to evaluate VC progression.
Results: Baseline dp-ucMGP was severely elevated in all groups. Initiation or continuation of VKAs further increased dp-ucMGP, whereas levels decreased in the rivaroxaban group and to a larger extent in the rivaroxaban+vitamin K2 group, but remained nevertheless elevated. Changes in coronary artery, thoracic aorta, and cardiac valve calcium scores and pulse wave velocity were not significantly different among the treatment arms. All cause death, stroke, and cardiovascular event rates were similar between the groups. Bleeding outcomes were not significantly different, except for a lower number of life-threatening and major bleeding episodes in the rivaroxaban arms versus the VKA arm.
Conclusions: Withdrawal of VKAs and high-dose vitamin K2 improve vitamin K status in patients on hemodialysis, but have no significant favorable effect on VC progression. Severe bleeding complications may be lower with rivaroxaban than with VKAs.
Keywords: direct oral anticoagulant; hemodialysis; oral anticoagulation; vascular calcification; vitamin K; vitamin K antagonist.
Copyright © 2020 by the American Society of Nephrology.
Figures

References
-
- De Vriese AS, Caluwé R, Raggi P: The atrial fibrillation conundrum in dialysis patients. Am Heart J 174: 111–119, 2016 - PubMed
-
- Van Der Meersch H, De Bacquer D, De Vriese AS: Vitamin K antagonists for stroke prevention in hemodialysis patients with atrial fibrillation: A systematic review and meta-analysis. Am Heart J 184: 37–46, 2017 - PubMed
-
- Chatrou ML, Winckers K, Hackeng TM, Reutelingsperger CP, Schurgers LJ: Vascular calcification: The price to pay for anticoagulation therapy with vitamin K-antagonists. Blood Rev 26: 155–166, 2012 - PubMed
-
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr., et al.: American College of Cardiology/American Heart Association Task Force on Practice Guidelines : 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 64: e1–e76, 2014 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

