NEXMIF/KIDLIA Knock-out Mouse Demonstrates Autism-Like Behaviors, Memory Deficits, and Impairments in Synapse Formation and Function
- PMID: 31704787
- PMCID: PMC6939493
- DOI: 10.1523/JNEUROSCI.0222-19.2019
NEXMIF/KIDLIA Knock-out Mouse Demonstrates Autism-Like Behaviors, Memory Deficits, and Impairments in Synapse Formation and Function
Abstract
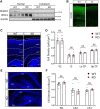
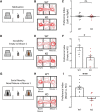
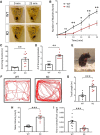
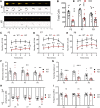
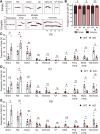
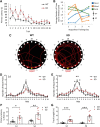
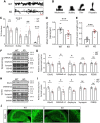
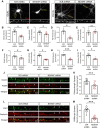
Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental disability that demonstrates impaired social interactions, communication deficits, and restrictive and repetitive behaviors. ASD has a strong genetic basis and many ASD-associated genes have been discovered thus far. Our previous work has shown that loss of expression of the X-linked gene NEXMIF/KIDLIA is implicated in patients with autistic features and intellectual disability (ID). To further determine the causal role of the gene in the disorder, and to understand the cellular and molecular mechanisms underlying the pathology, we have generated a NEXMIF knock-out (KO) mouse. We find that male NEXMIF KO mice demonstrate reduced sociability and communication, elevated repetitive grooming behavior, and deficits in learning and memory. Loss of NEXMIF/KIDLIA expression results in a significant decrease in synapse density and synaptic protein expression. Consistently, male KO animals show aberrant synaptic function as measured by excitatory miniatures and postsynaptic currents in the hippocampus. These findings indicate that NEXMIF KO mice recapitulate the phenotypes of the human disorder. The NEXMIF KO mouse model will be a valuable tool for studying the complex mechanisms involved in ASD and for the development of novel therapeutics for this disorder.SIGNIFICANCE STATEMENT Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental disorder characterized by behavioral phenotypes. Based on our previous work, which indicated the loss of NEXMIF/KIDLIA was associated with ASD, we generated NEXMIF knock-out (KO) mice. The NEXMIF KO mice demonstrate autism-like behaviors including deficits in social interaction, increased repetitive self-grooming, and impairments in communication and in learning and memory. The KO neurons show reduced synapse density and a suppression in synaptic transmission, indicating a role for NEXMIF in regulating synapse development and function. The NEXMIF KO mouse faithfully recapitulates the human disorder, and thus serves as an animal model for future investigation of the NEXMIF-dependent neurodevelopmental disorders.
Keywords: ASD; NEXMIF; autism spectrum disorder; brain development; synapse.
Copyright © 2020 the authors.
Figures


Similar articles
-
Replicable in vivo physiological and behavioral phenotypes of the Shank3B null mutant mouse model of autism.Mol Autism. 2017 Jun 15;8:26. doi: 10.1186/s13229-017-0142-z. eCollection 2017. Mol Autism. 2017. PMID: 28638591 Free PMC article.
-
Learning delays in a mouse model of Autism Spectrum Disorder.Behav Brain Res. 2016 Apr 15;303:201-7. doi: 10.1016/j.bbr.2016.02.006. Epub 2016 Feb 9. Behav Brain Res. 2016. PMID: 26873041 Free PMC article.
-
Heterozygous Nexmif female mice demonstrate mosaic NEXMIF expression, autism-like behaviors, and abnormalities in dendritic arborization and synaptogenesis.Heliyon. 2024 Jan 24;10(3):e24703. doi: 10.1016/j.heliyon.2024.e24703. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38322873 Free PMC article.
-
Autism Spectrum Disorders: Translating human deficits into mouse behavior.Neurobiol Learn Mem. 2015 Oct;124:71-87. doi: 10.1016/j.nlm.2015.07.013. Epub 2015 Jul 26. Neurobiol Learn Mem. 2015. PMID: 26220900 Review.
-
Behavioral phenotypes and neurobiological mechanisms in the Shank1 mouse model for autism spectrum disorder: A translational perspective.Behav Brain Res. 2018 Oct 15;352:46-61. doi: 10.1016/j.bbr.2017.09.038. Epub 2017 Sep 28. Behav Brain Res. 2018. PMID: 28963042 Review.
Cited by
-
Cortex-restricted deletion of Foxp1 impairs barrel formation and induces aberrant tactile responses in a mouse model of autism.Mol Autism. 2023 Sep 11;14(1):34. doi: 10.1186/s13229-023-00567-0. Mol Autism. 2023. PMID: 37691105 Free PMC article.
-
Prkn knockout mice show autistic-like behaviors and aberrant synapse formation.iScience. 2022 Jun 10;25(7):104573. doi: 10.1016/j.isci.2022.104573. eCollection 2022 Jul 15. iScience. 2022. PMID: 35789851 Free PMC article.
-
NEXMIF overexpression is associated with autism-like behaviors and alterations in dendritic arborization and spine formation in mice.Front Neurosci. 2025 Jun 18;19:1556570. doi: 10.3389/fnins.2025.1556570. eCollection 2025. Front Neurosci. 2025. PMID: 40606839 Free PMC article.
-
Nexmifa Regulates Axon Morphogenesis in Motor Neurons in Zebrafish.Front Mol Neurosci. 2022 Mar 31;15:848257. doi: 10.3389/fnmol.2022.848257. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35431796 Free PMC article.
-
Effects of chronic sleep restriction on the neuro-phenotypes of Ctnnd2 knockout mice.Brain Behav. 2023 Jul;13(7):e3075. doi: 10.1002/brb3.3075. Epub 2023 May 24. Brain Behav. 2023. PMID: 37226399 Free PMC article.
References
-
- Allen Institute for Brain Science (2004) Allen Mouse Brain Alatas. Experiment 69531127, Gene C77370. Available at brain-map.org/api/index.html.
-
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Robinson Rosenberg C, White T, Durkin MS, Imm P, Nikolaou L, Yeargin-Allsopp M, Lee LC, Harrington R, Lopez M, Fitzgerald RT, Hewitt A, Pettygrove S, et al. (2018) Prevalence of autism spectrum disorder among children aged 8 years: autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ 67:1–23. 10.15585/mmwr.ss6706a1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
