Universal scaling relations for the rational design of molecular water oxidation catalysts with near-zero overpotential
- PMID: 31704927
- PMCID: PMC6841662
- DOI: 10.1038/s41467-019-12994-w
Universal scaling relations for the rational design of molecular water oxidation catalysts with near-zero overpotential
Abstract
A major roadblock in realizing large-scale production of hydrogen via electrochemical water splitting is the cost and inefficiency of current catalysts for the oxygen evolution reaction (OER). Computational research has driven important developments in understanding and designing heterogeneous OER catalysts using linear scaling relationships derived from computed binding energies. Herein, we interrogate 17 of the most active molecular OER catalysts, based on different transition metals (Ru, Mn, Fe, Co, Ni, and Cu), and show they obey similar scaling relations to those established for heterogeneous systems. However, we find that the conventional OER descriptor underestimates the activity for very active OER complexes as the standard approach neglects a crucial one-electron oxidation that many molecular catalysts undergo prior to O-O bond formation. Importantly, this additional step allows certain molecular catalysts to circumvent the "overpotential wall", leading to enhanced performance. With this knowledge, we establish fundamental principles for the design of ideal molecular OER catalysts.
Conflict of interest statement
The authors declare no competing interests.
Figures




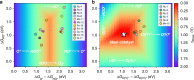
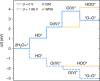

Similar articles
-
Toward Rational Design of Mononuclear Nickel Complexes as Water Oxidation Catalysts Exploring the Ligand Effects on the Rate-Determining Step.Chemphyschem. 2024 Oct 16;25(20):e202400533. doi: 10.1002/cphc.202400533. Epub 2024 Aug 15. Chemphyschem. 2024. PMID: 38925604
-
Metalloporphyrins as Catalytic Models for Studying Hydrogen and Oxygen Evolution and Oxygen Reduction Reactions.Acc Chem Res. 2022 Mar 15;55(6):878-892. doi: 10.1021/acs.accounts.1c00753. Epub 2022 Feb 22. Acc Chem Res. 2022. PMID: 35192330
-
A review on fundamentals for designing oxygen evolution electrocatalysts.Chem Soc Rev. 2020 Apr 7;49(7):2196-2214. doi: 10.1039/c9cs00607a. Chem Soc Rev. 2020. PMID: 32133479 Review.
-
Deciphering the impact of Zn-incorporation on M-NC (M = Fe, Co, Ni, Cu) type catalysts for enhanced HER and OER performance.Phys Chem Chem Phys. 2025 Apr 3;27(14):7240-7249. doi: 10.1039/d5cp00751h. Phys Chem Chem Phys. 2025. PMID: 40116329
-
Progress of Heterogeneous Iridium-Based Water Oxidation Catalysts.ACS Nano. 2022 Nov 22;16(11):17761-17777. doi: 10.1021/acsnano.2c08519. Epub 2022 Nov 10. ACS Nano. 2022. PMID: 36355040 Review.
Cited by
-
Harnessing the electronic structure of active metals to lower the overpotential of the electrocatalytic oxygen evolution reaction.Chem Sci. 2023 Dec 12;15(4):1348-1363. doi: 10.1039/d3sc05891c. eCollection 2024 Jan 24. Chem Sci. 2023. PMID: 38274069 Free PMC article.
-
Computational Analysis of Structure - Activity Relationships in Highly Active Homogeneous Ruthenium-based Water Oxidation Catalysts.Catalysts. 2022 Aug;12(8):863. doi: 10.3390/catal12080863. Epub 2022 Aug 5. Catalysts. 2022. PMID: 37309356 Free PMC article.
-
Immobilization of a [CoIIICoII(H2O)W11O39]7- Polyoxoanion for the Photocatalytic Oxygen Evolution Reaction.ACS Mater Au. 2022 Jul 13;2(4):505-515. doi: 10.1021/acsmaterialsau.2c00025. Epub 2022 May 25. ACS Mater Au. 2022. PMID: 35856075 Free PMC article.
-
Pivotal role of reversible NiO6 geometric conversion in oxygen evolution.Nature. 2022 Nov;611(7937):702-708. doi: 10.1038/s41586-022-05296-7. Epub 2022 Oct 26. Nature. 2022. PMID: 36289339
-
Principles of Water Electrolysis and Recent Progress in Cobalt-, Nickel-, and Iron-Based Oxides for the Oxygen Evolution Reaction.Angew Chem Int Ed Engl. 2022 Jan 3;61(1):e202103824. doi: 10.1002/anie.202103824. Epub 2021 Jul 21. Angew Chem Int Ed Engl. 2022. PMID: 34138511 Free PMC article. Review.
References
-
- Dannenberg A, Barrett S. Cooperating to avoid catastrophe. Nat. Hum. Behav. 2018;2:435–437. - PubMed
-
- Gray HB. Powering the planet with solar fuel. Nat. Chem. 2009;1:7. - PubMed
-
- Zhao G, Huang X, Wang X, Wang X. Progress in catalyst exploration for heterogeneous CO 2 reduction and utilization: a critical review. J. Mater. Chem. A. 2017;5:21625–21649.
-
- Vogt C, Monai M, Kramer GJ, Weckhuysen BM. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat. Catal. 2019;2:188–197.
-
- Suen N-T, et al. Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem. Soc. Rev. 2017;46:337–365. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

