Rare-earth (Gd3+,Yb3+/Tm3+, Eu3+) co-doped hydroxyapatite as magnetic, up-conversion and down-conversion materials for multimodal imaging
- PMID: 31705047
- PMCID: PMC6841688
- DOI: 10.1038/s41598-019-52885-0
Rare-earth (Gd3+,Yb3+/Tm3+, Eu3+) co-doped hydroxyapatite as magnetic, up-conversion and down-conversion materials for multimodal imaging
Abstract
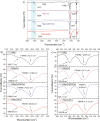
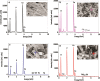
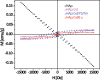
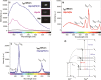
Taking advantage of the flexibility of the apatite structure, nano- and micro-particles of hydroxyapatite (HAp) were doped with different combinations of rare earth ions (RE3+ = Gd, Eu, Yb, Tm) to achieve a synergy among their magnetic and optical properties and to enable their application in preventive medicine, particularly diagnostics based on multimodal imaging. All powders were synthesized through hydrothermal processing at T ≤ 200 °C. An X-ray powder diffraction analysis showed that all powders crystallized in P63/m space group of the hexagonal crystal structure. The refined unit-cell parameters reflected a decrease in the unit cell volume as a result of the partial substitution of Ca2+ with smaller RE3+ ions at both cation positions. The FTIR analysis additionally suggested that a synergy may exist solely in the triply doped system, where the lattice symmetry and vibration modes become more coherent than in the singly or doubly doped systems. HAp:RE3+ optical characterization revealed a change in the energy band gap and the appearance of a weak blue luminescence (λex = 370 nm) due to an increased concentration of defects. The "up"- and the "down"-conversion spectra of HAp:Gd/Yb/Tm and HAp:Gd/Eu powders showed characteristic transitions of Tm3+ and Eu3+, respectively. Furthermore, in contrast to diamagnetic HAp, all HAp:RE3+ powders exhibited paramagnetic behavior. Cell viability tests of HAp:Gd/Yb/Tm and HAp:Gd/Eu powders in human dental pulp stem cell cultures indicated their good biocompatibility.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Habraken W, Habibovic P, Epple M, Bohner M. Calcium phosphates in biomedical applications: Materials for the future? Materials Today. 2016;19:69–87. doi: 10.1016/j.mattod.2015.10.008. - DOI
-
- Veselinović L, et al. Crystal structure of cobalt-substituted calcium hydroxyapatite nanopowders prepared by hydrothermal processing. J. Appl. Crystallogr. 2010;43:320–327. doi: 10.1107/S0021889809051395. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

