Zebrafish etv2 knock-in line labels vascular endothelial and blood progenitor cells
- PMID: 31705559
- PMCID: PMC7181369
- DOI: 10.1002/dvdy.130
Zebrafish etv2 knock-in line labels vascular endothelial and blood progenitor cells
Abstract
Background: ETS transcription factor Etv2/Etsrp is one of the earliest markers for vascular and hematopoietic progenitors and functions as a key regulator of hematovascular development in multiple vertebrates, including zebrafish. Therefore, transgenic etv2 reporter lines provide a valuable tool to study vasculogenesis and hematopoiesis. However, previously generated zebrafish reporter lines do not fully recapitulate the endogenous pattern of etv2 expression.
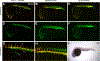
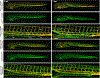
Results: Here we used CRISPR/Cas9-mediated homology-independent DNA repair approach to knock-in a Gal4 transcriptional activator into the zebrafish etv2 genomic locus, thus generating etv2 ci32Gt gene trap line. etv2 ci32Gt ; UAS:GFP embryos show GFP expression in vascular endothelial, myeloid and red blood cells. Because gal4 insertion interrupts the etv2 locus, homozygous etv2 ci32Gt embryos display defects in vasculogenesis and myelopoiesis, and enable visualizing etv2-deficient hematovascular progenitors in live embryos. Furthermore, we performed differential transcriptome analysis of sorted GFP-positive cells from heterozygous and homozygous etv2 ci32Gt embryos. Approximately 500 downregulated genes were identified in etv2 ci32Gt homozygous embryos, which include multiple genes expressed in vascular endothelial and myeloid cells.
Conclusions: The etv2 ci32Gt gene trap line and the data sets of misregulated genes will be valuable resources to study hematopoietic and vascular development.
Keywords: CRISPR; Cas9; RNA-seq; myeloid; red blood cell; transcriptome; vascular endothelial; zebrafish.
© 2019 Wiley Periodicals, Inc.
Figures
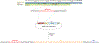






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

