Multidomain computational modeling of photoacoustic imaging: verification, validation, and image quality prediction
- PMID: 31705636
- PMCID: PMC7005568
- DOI: 10.1117/1.JBO.24.12.121910
Multidomain computational modeling of photoacoustic imaging: verification, validation, and image quality prediction
Abstract
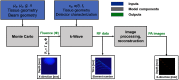
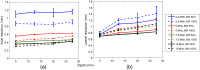
As photoacoustic imaging (PAI) technology matures, computational modeling will increasingly represent a critical tool for facilitating clinical translation through predictive simulation of real-world performance under a wide range of device and biological conditions. While modeling currently offers a rapid, inexpensive tool for device development and prediction of fundamental image quality metrics (e.g., spatial resolution and contrast ratio), rigorous verification and validation will be required of models used to provide regulatory-grade data that effectively complements and/or replaces in vivo testing. To address methods for establishing model credibility, we developed an integrated computational model of PAI by coupling a previously developed three-dimensional Monte Carlo model of tissue light transport with a two-dimensional (2D) acoustic wave propagation model implemented in the well-known k-Wave toolbox. We then evaluated ability of the model to predict basic image quality metrics by applying standardized verification and validation principles for computational models. The model was verified against published simulation data and validated against phantom experiments using a custom PAI system. Furthermore, we used the model to conduct a parametric study of optical and acoustic design parameters. Results suggest that computationally economical 2D acoustic models can adequately predict spatial resolution, but metrics such as signal-to-noise ratio and penetration depth were difficult to replicate due to challenges in modeling strong clutter observed in experimental images. Parametric studies provided quantitative insight into complex relationships between transducer characteristics and image quality as well as optimal selection of optical beam geometry to ensure adequate image uniformity. Multidomain PAI simulation tools provide high-quality tools to aid device development and prediction of real-world performance, but further work is needed to improve model fidelity, especially in reproducing image noise and clutter.
Keywords: Monte Carlo; image quality; k-Wave; photoacoustic imaging; simulation.
Figures








References
-
- Xia W., Steenbergen W., Manohar S., “Photoacoustic mammography: prospects and promises,” Breast Cancer Manag. 3(5), 387–390 (2014).10.2217/bmt.14.32 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

