CSGALNACT1-congenital disorder of glycosylation: A mild skeletal dysplasia with advanced bone age
- PMID: 31705726
- PMCID: PMC7027858
- DOI: 10.1002/humu.23952
CSGALNACT1-congenital disorder of glycosylation: A mild skeletal dysplasia with advanced bone age
Abstract
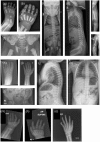
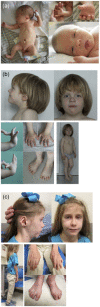
Congenital disorders of glycosylation (CDGs) comprise a large number of inherited metabolic defects that affect the biosynthesis and attachment of glycans. CDGs manifest as a broad spectrum of disease, most often including neurodevelopmental and skeletal abnormalities and skin laxity. Two patients with biallelic CSGALNACT1 variants and a mild skeletal dysplasia have been described previously. We investigated two unrelated patients presenting with short stature with advanced bone age, facial dysmorphism, and mild language delay, in whom trio-exome sequencing identified novel biallelic CSGALNACT1 variants: compound heterozygosity for c.1294G>T (p.Asp432Tyr) and the deletion of exon 4 that includes the start codon in one patient, and homozygosity for c.791A>G (p.Asn264Ser) in the other patient. CSGALNACT1 encodes CSGalNAcT-1, a key enzyme in the biosynthesis of sulfated glycosaminoglycans chondroitin and dermatan sulfate. Biochemical studies demonstrated significantly reduced CSGalNAcT-1 activity of the novel missense variants, as reported previously for the p.Pro384Arg variant. Altered levels of chondroitin, dermatan, and heparan sulfate moieties were observed in patients' fibroblasts compared to controls. Our data indicate that biallelic loss-of-function mutations in CSGALNACT1 disturb glycosaminoglycan synthesis and cause a mild skeletal dysplasia with advanced bone age, CSGALNACT1-CDG.
Keywords: CSGALNACT1-CDG; CSGalNAcT-1; advanced bone age; cartilage and brain development; glycosaminoglycan; joint laxity; macrocephaly; proteoglycan; short stature.
© 2019 The Authors. Human Mutation published by Wiley Periodicals, Inc.
Conflict of interest statement
N. K. is a distinguished Jean and George Brumley Professor and a paid consultant for and holds significant stock of Rescindo Therapeutics, Inc. Remaining authors declare that there are no conflict of interests.
Figures

References
-
- Bishop, J. R. , Schuksz, M. , & Esko, J. D. (2007). Heparan sulphate proteoglycans fine‐tune mammalian physiology. Nature, 446(7139), 1030–1037. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

