Non-invasive bioluminescence imaging of HCoV-OC43 infection and therapy in the central nervous system of live mice
- PMID: 31705922
- PMCID: PMC7114176
- DOI: 10.1016/j.antiviral.2019.104646
Non-invasive bioluminescence imaging of HCoV-OC43 infection and therapy in the central nervous system of live mice
Abstract
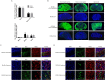
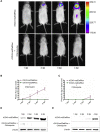
Human coronaviruses (HCoVs) are important pathogens that cause upper respiratory tract infections and have neuroinvasive abilities; however, little is known about the dynamic infection process of CoVs in vivo, and there are currently no specific antiviral drugs to prevent or treat HCoV infection. Here, we verified the replication ability and pathogenicity of a reporter HCoV-OC43 strain expressing Renilla luciferase (Rluc; rOC43-ns2DelRluc) in mice with different genetic backgrounds (C57BL/6 and BALB/c). Additionally, we monitored the spatial and temporal progression of HCoV-OC43 through the central nervous system (CNS) of live BALB/c mice after intranasal or intracerebral inoculation with rOC43-ns2DelRluc. We found that rOC43-ns2DelRluc was fatal to suckling mice after intranasal inoculation, and that viral titers and Rluc expression were detected in the brains and spinal cords of mice infected with rOC43-ns2DelRluc. Moreover, viral replication was initially observed in the brain by non-invasive bioluminescence imaging before the infection spread to the spinal cord of BALB/c mice, consistent with its tropism in the CNS. Furthermore, the Rluc readout correlated with the HCoV replication ability and protein expression, which allowed quantification of antiviral activity in live mice. Additionally, we validated that chloroquine strongly inhibited rOC43-ns2DelRluc replication in vivo. These results provide new insights into the temporal and spatial dissemination of HCoV-OC43 in the CNS, and our methods provide an extremely sensitive platform for evaluating the efficacy of antiviral therapies to treat neuroinvasive HCoVs in live mice.
Keywords: Bioluminescence imaging; Chloroquine; Coronavirus; Mouse; Therapy.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no commercial or financial conflicts of interest.
Figures



References
-
- Adams M.J., Lefkowitz E.J., King A.M., Harrach B., Harrison R.L., Knowles N.J., Kropinski A.M., Krupovic M., Kuhn J.H., Mushegian A.R., Nibert M., Sabanadzovic S., Sanfacon H., Siddell S.G., Simmonds P., Varsani A., Zerbini F.M., Gorbalenya A.E., Davison A.J. Ratification vote on taxonomic proposals to the international committee on taxonomy of viruses (2016) Arch. Virol. 2016;161:2921–2949. doi: 10.1007/s00705-016-2977-6. - DOI - PMC - PubMed
-
- Arabi Y.M., Harthi A., Hussein J., Bouchama A., Johani S., Hajeer A.H., Saeed B.T., Wahbi A., Saedy A., AlDabbagh T., Okaili R., Sadat M., Balkhy H. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

