Alirocumab therapy in individuals with type 2 diabetes mellitus and atherosclerotic cardiovascular disease: analysis of the ODYSSEY DM-DYSLIPIDEMIA and DM-INSULIN studies
- PMID: 31706300
- PMCID: PMC6842201
- DOI: 10.1186/s12933-019-0951-9
Alirocumab therapy in individuals with type 2 diabetes mellitus and atherosclerotic cardiovascular disease: analysis of the ODYSSEY DM-DYSLIPIDEMIA and DM-INSULIN studies
Abstract
Background: Individuals with diabetes often have high levels of atherogenic lipoproteins and cholesterol reflected by elevated low-density lipoprotein cholesterol (LDL-C), non-high-density lipoprotein cholesterol (non-HDL-C), apolipoprotein B (ApoB), and LDL particle number (LDL-PN). The presence of atherosclerotic cardiovascular disease (ASCVD) increases the risk of future cardiovascular events. We evaluated the efficacy and safety of the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, alirocumab, among individuals with type 2 diabetes (T2DM), high LDL-C or non-HDL-C, and established ASCVD receiving maximally tolerated statin in ODYSSEY DM-DYSLIPIDEMIA (NCT02642159) and DM-INSULIN (NCT02585778).
Methods: In DM-DYSLIPIDEMIA, individuals with T2DM and mixed dyslipidemia (non-HDL-C ≥ 100 mg/dL; n = 413) were randomized to open-label alirocumab 75 mg every 2 weeks (Q2W) or usual care (UC) for 24 weeks, with UC options selected before stratified randomization. In DM-INSULIN, insulin-treated individuals with T2DM (LDL-C ≥ 70 mg/dL; n = 441) were randomized in a double-blind fashion to alirocumab 75 mg Q2W or placebo for 24 weeks. Study participants also had a glycated hemoglobin < 9% (DM-DYSLIPIDEMIA) or < 10% (DM-INSULIN). Alirocumab dose was increased to 150 mg Q2W at week 12 if week 8 LDL-C was ≥ 70 mg/dL (DM-INSULIN) or non-HDL-C was ≥ 100 mg/dL (DM-DYSLIPIDEMIA). Lipid reductions and safety were assessed in patients with ASCVD from these studies.
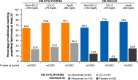
Results: This analysis included 142 DM-DYSLIPIDEMIA and 177 DM-INSULIN participants with ASCVD, including 95.1% and 86.4% with coronary heart disease, and 32.4% and 49.7% with microvascular diabetes complications, respectively. At week 24, alirocumab significantly reduced LDL-C, non-HDL-C, ApoB, and LDL-PN from baseline versus control. This translated into a greater proportion of individuals achieving non-HDL-C < 100 mg/dL (64.6% alirocumab/23.8% UC [DM-DYSLIPIDEMIA]; 65.4% alirocumab/14.9% placebo [DM-INSULIN]) and ApoB < 80 mg/dL (75.1% alirocumab/35.4% UC and 76.8% alirocumab/24.8% placebo, respectively) versus control at week 24 (all P < 0.0001). In pooling these studies, 66.4% (alirocumab) and 67.0% (control) of individuals reported treatment-emergent adverse events. The adverse event pattern was similar with alirocumab versus controls.
Conclusions: Among individuals with T2DM and ASCVD who had high non-HDL-C/LDL-C levels despite maximally tolerated statin, alirocumab significantly reduced atherogenic cholesterol and LDL-PN versus control. Alirocumab was generally well tolerated. Trial registration Clinicaltrials.gov. NCT02642159. Registered 30 December 2015 and Clinicaltrials.gov. NCT02585778. Registered 23 October 2015.
Keywords: Alirocumab; Atherosclerotic cardiovascular disease; Dyslipidemia; Low-density lipoprotein cholesterol; Type 2 diabetes mellitus.
Conflict of interest statement
Kausik K. Ray has received significant research grants from Pfizer Inc., Amgen, Sanofi, Regeneron Pharmaceuticals, Inc., and MSD Pharma outside of the submitted work; modest honoraria from Dr. Reddys, Zuellig Pharma, Sanofi, Amgen, Boehringer Ingelheim, Novo Nordisk, and Pfizer Inc.; and modest consultant/advisory board fees from Medco, AstraZeneca, Resverlogix, Kowa, Abbvie, Sanofi, Amgen, Boehringer Ingelheim, Esperion, Akcea, and Regeneron Pharmaceuticals, Inc. Stefano Del Prato has received research funding from AstraZeneca, Boehringer Ingelheim, Novartis Pharmaceuticals Co., and Merck Sharpe & Dohme; and is a consultant for or has received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, Laboratoires Servier, Merck Sharpe & Dohme, Novartis Pharmaceuticals Co., Novo Nordisk, Sanofi, Servier, and Takeda Pharmaceuticals. Dirk Müller-Wieland has received speaker’s bureau and consultant/advisory board fees from Amgen, AstraZeneca, Boehringer Ingelheim, MSD (Merck), Novartis, Novo Nordisk, and Sanofi. Bertrand Cariou has received research funding and honoraria from Amgen, Sanofi and Regeneron Pharmaceuticals, Inc.; research funding from Pfizer; and honoraria from Abbott, Amgen, Akcea, AstraZeneca, Pierre Fabre, Genfit, Eli Lilly and Company, MSD Merck & Co., Novo Nordisk, and Servier. Helen M. Colhoun has received grants, personal fees, and non-financial support from Sanofi and Regeneron Pharmaceuticals, Inc.; grants, institutional fees, and non-financial support from Eli Lilly and Company; grants from AstraZeneca and Pfizer Inc.; and grants and fees from Novo Nordisk; and owns stock in Roche Pharmaceuticals and Bayer. Francisco J. Tinahones has received speaker’s bureau and consultant/advisory board fees from AstraZeneca, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, Merck Sharpe & Dohme, Novartis Pharmaceuticals Co., Novo Nordisk, Sanofi, and Regeneron Pharmaceuticals, Inc. Catherine Domenger is an employee and shareholder in Sanofi. Alexia Letierce is an employee of and shareholder in Sanofi. Jonas Mandel is an employee of IviData Stats, contracted to Sanofi. Rita Samuel is an employee of and shareholder in Regeneron Pharmaceuticals, Inc. Maja Bujas-Bobanovic is an employee and shareholder in Sanofi. Lawrence A. Leiter has received personal fees from Esperion and HLS; grants and personal fees from Amgen, AstraZeneca, Eli Lilly and Company, Merck, Regeneron Pharmaceuticals, Inc., and Sanofi; and grants from Kowa and The Medicines Company.
Figures

References
-
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. - DOI - PubMed
-
- Ray KK, Leiter LA, Muller-Wieland D, et al. Alirocumab vs usual lipid-lowering care as add-on to statin therapy in individuals with type 2 diabetes and mixed dyslipidaemia: the ODYSSEY DM-DYSLIPIDEMIA randomized trial. Diabetes Obes Metab. 2018;20:1479–1489. doi: 10.1111/dom.13257. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

