The model of local axon homeostasis - explaining the role and regulation of microtubule bundles in axon maintenance and pathology
- PMID: 31706327
- PMCID: PMC6842214
- DOI: 10.1186/s13064-019-0134-0
The model of local axon homeostasis - explaining the role and regulation of microtubule bundles in axon maintenance and pathology
Abstract
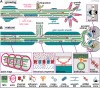
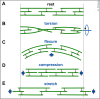
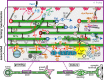
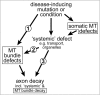
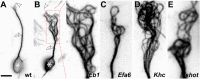
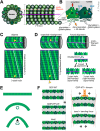
Axons are the slender, cable-like, up to meter-long projections of neurons that electrically wire our brains and bodies. In spite of their challenging morphology, they usually need to be maintained for an organism's lifetime. This makes them key lesion sites in pathological processes of ageing, injury and neurodegeneration. The morphology and physiology of axons crucially depends on the parallel bundles of microtubules (MTs), running all along to serve as their structural backbones and highways for life-sustaining cargo transport and organelle dynamics. Understanding how these bundles are formed and then maintained will provide important explanations for axon biology and pathology. Currently, much is known about MTs and the proteins that bind and regulate them, but very little about how these factors functionally integrate to regulate axon biology. As an attempt to bridge between molecular mechanisms and their cellular relevance, we explain here the model of local axon homeostasis, based on our own experiments in Drosophila and published data primarily from vertebrates/mammals as well as C. elegans. The model proposes that (1) the physical forces imposed by motor protein-driven transport and dynamics in the confined axonal space, are a life-sustaining necessity, but pose a strong bias for MT bundles to become disorganised. (2) To counterbalance this risk, MT-binding and -regulating proteins of different classes work together to maintain and protect MT bundles as necessary transport highways. Loss of balance between these two fundamental processes can explain the development of axonopathies, in particular those linking to MT-regulating proteins, motors and transport defects. With this perspective in mind, we hope that more researchers incorporate MTs into their work, thus enhancing our chances of deciphering the complex regulatory networks that underpin axon biology and pathology.
Keywords: Drosophila; actin; axons; cytoskeleton; microtubules; neurodegeneration.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
How neurons maintain their axons long-term: an integrated view of axon biology and pathology.Front Neurosci. 2023 Jul 26;17:1236815. doi: 10.3389/fnins.2023.1236815. eCollection 2023. Front Neurosci. 2023. PMID: 37564364 Free PMC article. Review.
-
The intricate relationship between microtubules and their associated motor proteins during axon growth and maintenance.Neural Dev. 2013 Sep 8;8:17. doi: 10.1186/1749-8104-8-17. Neural Dev. 2013. PMID: 24010872 Free PMC article. Review.
-
A conceptual view at microtubule plus end dynamics in neuronal axons.Brain Res Bull. 2016 Sep;126(Pt 3):226-237. doi: 10.1016/j.brainresbull.2016.08.006. Epub 2016 Aug 12. Brain Res Bull. 2016. PMID: 27530065 Free PMC article. Review.
-
Efa6 protects axons and regulates their growth and branching by inhibiting microtubule polymerisation at the cortex.Elife. 2019 Nov 13;8:e50319. doi: 10.7554/eLife.50319. Elife. 2019. PMID: 31718774 Free PMC article.
-
Periodic actin structures in neuronal axons are required to maintain microtubules.Mol Biol Cell. 2017 Jan 15;28(2):296-308. doi: 10.1091/mbc.E16-10-0727. Epub 2016 Nov 23. Mol Biol Cell. 2017. PMID: 27881663 Free PMC article.
Cited by
-
The node of Ranvier influences the in vivo axonal transport of mitochondria and signaling endosomes.iScience. 2024 Oct 11;27(11):111158. doi: 10.1016/j.isci.2024.111158. eCollection 2024 Nov 15. iScience. 2024. PMID: 39524336 Free PMC article.
-
A kinesin-1 variant reveals motor-induced microtubule damage in cells.Curr Biol. 2022 Jun 6;32(11):2416-2429.e6. doi: 10.1016/j.cub.2022.04.020. Epub 2022 May 2. Curr Biol. 2022. PMID: 35504282 Free PMC article.
-
How neurons maintain their axons long-term: an integrated view of axon biology and pathology.Front Neurosci. 2023 Jul 26;17:1236815. doi: 10.3389/fnins.2023.1236815. eCollection 2023. Front Neurosci. 2023. PMID: 37564364 Free PMC article. Review.
-
Modeling links softening of myelin and spectrin scaffolds of axons after a concussion to increased vulnerability to repeated injuries.Proc Natl Acad Sci U S A. 2021 Jul 13;118(28):e2024961118. doi: 10.1073/pnas.2024961118. Proc Natl Acad Sci U S A. 2021. PMID: 34234016 Free PMC article.
-
Causes, costs and consequences of kinesin motors communicating through the microtubule lattice.J Cell Sci. 2023 Mar 1;136(5):jcs260735. doi: 10.1242/jcs.260735. Epub 2023 Mar 3. J Cell Sci. 2023. PMID: 36866642 Free PMC article.
References
-
- Hoffman PN. Review : The Synthesis, Axonal Transport, and Phosphorylation of Neurofilaments Determine Axonal Caliber in Myelinated Nerve Fibers. Neuroscientist. 1995;1:76–83. doi: 10.1177/107385849500100204. - DOI
-
- Bichenback J. International perspectives on spinal cord injury. WHO, ISCOS: Switzerland; 2013.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

