TonEBP-deficiency accelerates intervertebral disc degeneration underscored by matrix remodeling, cytoskeletal rearrangements, and changes in proinflammatory gene expression
- PMID: 31707045
- PMCID: PMC7078052
- DOI: 10.1016/j.matbio.2019.10.007
TonEBP-deficiency accelerates intervertebral disc degeneration underscored by matrix remodeling, cytoskeletal rearrangements, and changes in proinflammatory gene expression
Abstract
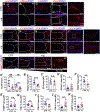
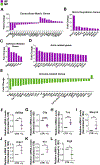
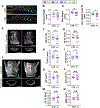
The tonicity-responsive enhancer binding protein (TonEBP) plays an important role in intervertebral disc and axial skeleton embryogenesis. However, the contribution of this osmoregulatory transcription factor in postnatal intervertebral disc homeostasis is not known in vivo. Here, we show for the first time that TonEBP-deficient mice have pronounced age-related degeneration of the intervertebral disc with annular and endplate herniations. Using FTIR-imaging spectroscopy, quantitative immunohistochemistry, and tissue-specific transcriptomic analysis, we provide morphological and molecular evidence that the overall phenotype is driven by a replacement of water-binding proteoglycans with fibrocartilaginous matrix. Whereas TonEBP deficiency in the AF compartment caused tissue fibrosis associated with alterations in actin cytoskeleton and adhesion molecules, predominant changes in pro-inflammatory pathways were seen in the NP compartment of mutants, underscoring disc compartment-specific effects. Additionally, TonEBP-deficient mice presented with compromised trabecular bone properties of vertebrae. These results provide the first in vivo support to the long-held hypothesis that TonEBP is crucial for postnatal homeostasis of the spine and controls a multitude of functions in addition to cellular osmoadaptation.
Keywords: Actin cytoskeleton; Inflammation; Intervertebral disc; Matrix remodeling; TonEBP.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures




References
-
- Ghosh P, Taylor TK, Horsburgh BA. (1975) The composition and protein metabolism in the immature rabbit intervertebral disc. Cell Tissue Res 163:223–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

