Deconstructing tissue engineered trachea: Assessing the role of synthetic scaffolds, segmental replacement and cell seeding on graft performance
- PMID: 31707085
- PMCID: PMC7231426
- DOI: 10.1016/j.actbio.2019.11.008
Deconstructing tissue engineered trachea: Assessing the role of synthetic scaffolds, segmental replacement and cell seeding on graft performance
Abstract
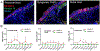
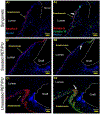
The ideal construct for tracheal replacement remains elusive in the management of long segment airway defects. Tissue engineered tracheal grafts (TETG) have been limited by the development of graft stenosis or collapse, infection, or lack of an epithelial lining. We applied a mouse model of orthotopic airway surgery to assess the impact of three critical barriers encountered in clinical applications: the scaffold, the extent of intervention, and the impact of cell seeding and characterized their impact on graft performance. First, synthetic tracheal scaffolds electrospun from polyethylene terephthalate / polyurethane (PET/PU) were orthotopically implanted in anterior tracheal defects of C57BL/6 mice. Scaffolds demonstrated complete coverage with ciliated respiratory epithelium by 2 weeks. Epithelial migration was accompanied by macrophage infiltration which persisted at long term (>6 weeks) time points. We then assessed the impact of segmental tracheal implantation using syngeneic trachea as a surrogate for the ideal tracheal replacement. Graft recovery involved local upregulation of epithelial progenitor populations and there was no evidence of graft stenosis or necrosis. Implantation of electrospun synthetic tracheal scaffold for segmental replacement resulted in respiratory distress and required euthanasia at an early time point. There was limited epithelial coverage of the scaffold with and without seeded bone marrow-derived mononuclear cells (BM-MNCs). We conclude that synthetic scaffolds support re-epithelialization in orthotopic patch implantation, syngeneic graft integration occurs with focal repair mechanisms, however epithelialization in segmental synthetic scaffolds is limited and is not influenced by cell seeding. STATEMENT OF SIGNIFICANCE: The life-threatening nature of long-segment tracheal defects has led to clinical use of tissue engineered tracheal grafts in the last decade for cases of compassionate use. However, the ideal tracheal reconstruction using tissue-engineered tracheal grafts (TETG) has not been clarified. We addressed the core challenges in tissue engineered tracheal replacement (re-epithelialization and graft patency) by defining the role of cell seeding with autologous bone marrow-derived mononuclear cells, the mechanism of respiratory epithelialization and proliferation, and the role of the inflammatory immune response in regeneration. This research will facilitate comprehensive understanding of cellular regeneration and neotissue formation on TETG, which will permit targeted therapies for accelerating re-epithelialization and attenuating stenosis in tissue engineered airway replacement.
Keywords: Bone marrow mononuclear cells; Epithelialization; Syngeneic segmental tracheal transplantation; Synthetic PET/PU scaffold; Tissue engineered trachea graft.
Copyright © 2019. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of conflict of interest
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Jed Johnson is a co-founder and chief technology officer of Nanofiber Solutions, Inc. Christopher Breuer receives research support from Cook Medical (Bloomington, Indiana, USA) and Gunze Ltd (Kyoto, Japan). The remaining authors have no disclosures.
Figures


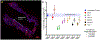


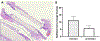

Similar articles
-
Mouse Model of Tracheal Replacement With Electrospun Nanofiber Scaffolds.Ann Otol Rhinol Laryngol. 2019 May;128(5):391-400. doi: 10.1177/0003489419826134. Epub 2019 Jan 30. Ann Otol Rhinol Laryngol. 2019. PMID: 30700095 Free PMC article.
-
Seeding and Implantation of a Biosynthetic Tissue-engineered Tracheal Graft in a Mouse Model.J Vis Exp. 2019 Apr 1;(146):10.3791/59173. doi: 10.3791/59173. J Vis Exp. 2019. PMID: 30985752 Free PMC article.
-
Factors Influencing Poor Outcomes in Synthetic Tissue-Engineered Tracheal Replacement.Otolaryngol Head Neck Surg. 2019 Sep;161(3):458-467. doi: 10.1177/0194599819844754. Epub 2019 Apr 30. Otolaryngol Head Neck Surg. 2019. PMID: 31035858 Free PMC article.
-
Clinical Translation of Tissue Engineered Trachea Grafts.Ann Otol Rhinol Laryngol. 2016 Nov;125(11):873-885. doi: 10.1177/0003489416656646. Epub 2016 Jul 12. Ann Otol Rhinol Laryngol. 2016. PMID: 27411362 Free PMC article. Review.
-
Challenges and Opportunities in Developing Tracheal Substitutes for the Recovery of Long-Segment Defects.Macromol Biosci. 2024 Sep;24(9):e2400054. doi: 10.1002/mabi.202400054. Epub 2024 Jul 15. Macromol Biosci. 2024. PMID: 39008817 Review.
Cited by
-
Application of tissue engineering techniques in tracheal repair: a bibliometric study.Bioengineered. 2023 Dec;14(1):2274150. doi: 10.1080/21655979.2023.2274150. Epub 2023 Nov 6. Bioengineered. 2023. PMID: 37927226 Free PMC article.
-
Successful Early Neovascularization in Composite Tracheal Grafts.Otolaryngol Head Neck Surg. 2023 Oct;169(4):1035-1040. doi: 10.1002/ohn.350. Epub 2023 Apr 10. Otolaryngol Head Neck Surg. 2023. PMID: 37036314 Free PMC article. Clinical Trial.
-
Long-Term Chondrocyte Retention in Partially Decellularized Tracheal Grafts.Otolaryngol Head Neck Surg. 2024 Jan;170(1):239-244. doi: 10.1002/ohn.409. Epub 2023 Jun 27. Otolaryngol Head Neck Surg. 2024. PMID: 37365963 Free PMC article.
-
Biocompatible Materials in Otorhinolaryngology and Their Antibacterial Properties.Int J Mol Sci. 2022 Feb 25;23(5):2575. doi: 10.3390/ijms23052575. Int J Mol Sci. 2022. PMID: 35269718 Free PMC article. Review.
-
Dynamic flow for efficient partial decellularization of tracheal grafts: A preliminary rabbit study.Laryngoscope Investig Otolaryngol. 2024 Apr 13;9(2):e1247. doi: 10.1002/lio2.1247. eCollection 2024 Apr. Laryngoscope Investig Otolaryngol. 2024. PMID: 38618643 Free PMC article.
References
-
- Park JH, Hong JM, Ju YM, Jung JW, Kang H-W, Lee SJ, Yoo JJ, Kim SW, Kim SH, Cho D-W, A novel tissue-engineered trachea with a mechanical behavior similar to native trachea, Biomaterials 62 (2015) 106–115. - PubMed
-
- Xu Y, Li D, Yin Z, He A, Lin M, Jiang G, Song X, Hu X, Liu Y, Wang J, Tissue-engineered trachea regeneration using decellularized trachea matrix treated with laser micropore technique, Acta biomaterialia 58 (2017) 113–121. - PubMed
-
- Kang Y, Wang C, Qiao Y, Gu J, Zhang H, Peijs T, Kong J, Zhang G, Shi X, Tissue-engineered trachea consisting of electrospun patterned sc-PLA/GO-g-IL fibrous membranes with antibacterial property and 3D-printed skeletons with elasticity, Biomacromolecules 20(4) (2019) 1765–1776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

