Incomplete Retinal Pigment Epithelial and Outer Retinal Atrophy in Age-Related Macular Degeneration: Classification of Atrophy Meeting Report 4
- PMID: 31708275
- PMCID: PMC7218279
- DOI: 10.1016/j.ophtha.2019.09.035
Incomplete Retinal Pigment Epithelial and Outer Retinal Atrophy in Age-Related Macular Degeneration: Classification of Atrophy Meeting Report 4
Abstract
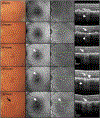
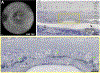
Purpose: To describe the defining features of incomplete retinal pigment epithelium (RPE) and outer retinal atrophy (iRORA), a consensus term referring to the OCT-based anatomic changes often identified before the development of complete RPE and outer retinal atrophy (cRORA) in age-related macular degeneration (AMD). We provide descriptive OCT and histologic examples of disease progression.
Design: Consensus meeting.
Participants: Panel of retina specialists, including retinal imaging experts, reading center leaders, and retinal histologists.
Methods: As part of the Classification of Atrophy Meeting (CAM) program, an international group of experts analyzed and discussed longitudinal multimodal imaging of eyes with AMD. Consensus was reached on a classification system for OCT-based structural alterations that occurred before the development of atrophy secondary to AMD. New terms of iRORA and cRORA were defined. This report describes in detail the CAM consensus on iRORA.
Main outcome measures: Defining the term iRORA through OCT imaging and longitudinal cases showing progression of atrophy, with histologic correlates.
Results: OCT was used in cases of early and intermediate AMD as the base imaging method to identify cases of iRORA. In the context of drusen, iRORA is defined on OCT as (1) a region of signal hypertransmission into the choroid, (2) a corresponding zone of attenuation or disruption of the RPE, and (3) evidence of overlying photoreceptor degeneration. The term iRORA should not be used when there is an RPE tear. Longitudinal studies confirmed the concept of progression from iRORA to cRORA.
Conclusions: An international consensus classification for OCT-defined anatomic features of iRORA are described and examples of longitudinal progression to cRORA are provided. The ability to identify these OCT changes reproducibly is essential to understand better the natural history of the disease, to identify high-risk signs of progression, and to study early interventions. Longitudinal data are required to quantify the implied risk of vision loss associated with these terms. The CAM classification provides initial definitions to enable these future endeavors, acknowledging that the classification will be refined as new data are generated.
Copyright © 2019 American Academy of Ophthalmology. All rights reserved.
Conflict of interest statement
Conflict of interest:
Dr. Guymer reports grants and personal fees from Novartis, personal fees from Bayer, Apellis, personal fees from Roche/Genentech, outside the submitted work.
Dr. Rosenfeld reports grants and personal fees from Carl Zeiss Meditec during the conduct of the study. He also received additional grant support from Genentech, and Tyrogenex. He has also received personal fees from Achillion Pharmaceuticals, Boehringer-Ingelheim, Carl Zeiss Meditec, Chengdu Kanghong Biotech, Healios K.K, Hemera Biosciences, F. Hoffmann-La Roche Ltd., Isarna Pharmaceuticals, Lin Bioscience, NGM Biopharmaceuticals, Ocunexus Therapeutics, Ocudyne, and Unity Biotechnology. Dr. Rosenfeld has equity interest in Apellis, Verana Health, and Ocudyne., outside the submitted work.
Dr. Curcio reports grants from Hoffman-LaRoche and Heidelberg Engineering.
Dr. Holz reports grants and personal fees from Heidelberg Engineering, grants and personal fees from Optos, grants from Zeiss, during the conduct of the study; grants and personal fees from Novartis, grants and personal fees from Bayer Healthcare, grants and personal fees from Genentech, grants and personal fees from Acucela, personal fees from Boehringer Ingelheim, grants and personal fees from Alcon, grants and personal fees from Allergan, outside the submitted work
Dr. Staurenghi reports personal fees and other from Heidelberg Engineering, grants, personal fees and other from Zeiss Meditec, grants from Optovue, grants and other from Optos, grants, personal fees and other from Centervue, grants from Nidek, grants, personal fees and other from Novartis, personal fees and other from Bayer, other from Boeheringer, other from Allergan, other from Alcon, outside the submitted work
Dr. Freund reports grants and personal fees from Genentech/Roche, personal fees from Heidelberg Engineering, personal fees from Optovue, personal fees from Allergan, personal fees from Novartis, personal fees from Carl Zeiss Meditec, during the conduct of the study.
Dr. Schmitz-Valckenberg reports grants from Acucela, grants and personal fees from Alcon/Novartis, grants and personal fees from Allergan, grants and personal fees from Bayer, grants and personal fees from Bioeq/Formycon, grants, personal fees and non-financial support from Carl Zeiss MediTec AG, grants and non-financial support from Centervue, personal fees from Galimedix, grants and non-financial support from Heidelberg Engineering, grants from Katairo, non-financial support from Optos, outside the submitted work.
Dr Sparrow reports grants from National Eye Institute (RO1EY024091, RO1EY12951. R24 EY027285, P30EY019007) Foundation Fighting Blindness, Research to Prevent Blindness, Heidelberg Engineering, Edward N and Della L. Thome Foundation; the Arnold and Mabel Beckman Initiative for Macular Research; Alcon, Alimera, Janssen Research and Development LLC, Baxter Healthcare Corporation, Othera Pharmaceuticals, Inc; personal fees from Bayer Healthcare, Pfizer; Astellas; outside the submitted work,
Dr. Spaide reports consulting fees and royalties from Topcon Medical Systems, consulting fees from Heidelberg Engineering, and royalties from DORC, outside the submitted work.
Dr. Tufail reports grants and personal fees from Novartis, personal fees from Roche, grants and personal fees from Bayer Healthcare, , personal fees from Allergan, grants and personal fees from Alcon, and personal fees from Heidelberg Engineering, personal feed from Kanghong, outside the submitted work. Supported in part from the National Institute for Health Research Biomedical Research Centre Moorfields Eye Hospital,.
Dr. Chakravarthy reports personal fees from Novartis, Bayer, Allergan and Heidelberg Engineeering.
Dr. Jaffe reports personal fees from Heidelberg Engineering, outside the submitted work.
Dr. Csaky reports personal fees from Genentech, Regeneron, Heidelberg Engineering, Gyroscope, Roche, Allergan, personal fees and grants from Ophthotech, Acucela, and equity interest in Apellis outside the submitted work..
Dr. Sarraf reports grants and other from Genentech, grants from Heidelberg, grants from Regeneron, grants and other from Optovue, and grants from Topcon, personal fees from Bayer and Novartis and Optovue, outside the submitted work.
Dr. Monés reports grants from Eyerisk Consortium 2020, Novartis, Bayer, Alcon, Roche, Ophthotech, personal consultation fees from Novartis, Bayer, Alcon, Roche, Genentech, Cellcure, Reneuron and stock shareholder from ophthotech and Notalvision
Dr. Tadayoni reports grants and personal fees from Novartis, grants and personal fees from Bayer, grants and personal fees from Allergan, personal fees from Roche-Genetech, personal fees from Thea, personal fees from Alcon, grants from Zeiss, personal fees from Oculis, outside the submitted work;.
Dr. Grunwald has nothing to disclose.
Dr. Bottoni reports personal fees from Novartis, personal fees from Bayer, non-financial support from Allergan, non-financial support from Heidelberg Engineering, outside the submitted work
Dr. Liakopoulos reports personal fees and non-financial support from Heidelberg Engineering and Carl Zeiss Meditec, personal fees from Novartis, personal fees from Allergan, personal fees from Bayer, outside the submitted work.
Dr. Pauleikhoff discloses participation in clinical studies financed by Roche, Novartis and Bayer and consultation fees of Novartis and Bayer.
Dr Pagliarini reports personal fees and non-financial support from Novartis, Bayer, Allergan, Alcon, Heidelberg Engineering and Zeiss, outside of the submitted work.
Dr. Chew has nothing to disclose
Dr. Viola, has nothing to disclose
Dr. Fleckenstein reports grants, personal fees and non-financial support from Heidelberg Engineering, non-financial support from Zeiss Meditech, grants and non-financial support from Optos, personal fees from Novartis, personal fees from Bayer, grants and personal fees from Genentech, from Roche, outside the submitted work; In addition, Dr. Fleckenstein has a patent US20140303013 A1 pending
Dr. Blodi has nothing to disclose
Dr Lim reports non-financial support from Novartis and Heidelberg Engineering.
Dr Chong is an Boehringer Ingelheim International GmBH.
Dr Lutty has nothing to disclose
Dr. Bird has nothing to disclose.
Dr. Sadda reports grants and other from Optos, grants and other from Carl Zeiss Meditec, during the conduct of the study; grants and other from Allergan, grants and other from Carl Zeiss Meditec, other from Alcon, other from Allergan, other from Genentech, other from Regeneron, other from Novartis, outside the submitted work.
Figures









References
-
- Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367–74 - PubMed
-
- Schmitz-Valckenberg S The Journey of “Geographic Atrophy” through Past, Present, and Future. Ophthalmologica. 2017;237:11–20 - PubMed
-
- Holz FG, Strauss EC, Schmitz-Valckenberg S, et al. Geographic Atrophy: Clinical Features and Potential Therapeutic Approaches. Ophthalmology. 2014;121:1079–91 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

