Dynamic Regulation of Adult-Specific Functions of the Nervous System by Signaling from the Reproductive System
- PMID: 31708396
- PMCID: PMC6907730
- DOI: 10.1016/j.cub.2019.10.011
Dynamic Regulation of Adult-Specific Functions of the Nervous System by Signaling from the Reproductive System
Abstract
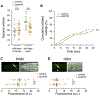
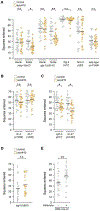
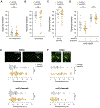
Unlike juveniles, adult animals engage in suites of behaviors related to the search for and selection of potential mates and mating, including appropriate responses to sex pheromones. As in other species [1], male sex pheromones modulate several behaviors and physiological processes in C. elegans hermaphrodites [2-5]. In particular, one of these small-molecule signals, an ascaroside ascr#10, causes reduced exploration, more avid mating, and improved reproductive performance (see the accompanying paper by Aprison and Ruvinsky in this issue of Current Biology) [6]. Here, we investigated the mechanism that restricts pheromone response to adult hermaphrodites. Unexpectedly, we found that attainment of developmental adulthood was not alone sufficient for the behavioral response to the pheromone. To modify exploratory behavior in response to male pheromone, adult hermaphrodites also require functional germline and egg-laying apparatus. We show that this dependence of behavior on the reproductive system is due to feedback from the vulva muscles that reports ongoing reproduction to the nervous system. Our results reveal an activity-dependent conduit by which the reproductive system continuously licenses adult behaviors, including appropriate responses to the pheromones of the opposite sex. More broadly, our results suggest that signals from peripheral organs may serve as an important component of assuring age-appropriate functions of the nervous system.
Keywords: C. elegans; adulthood; behavioral maturation; exploratory behavior; neuromodulation; neuronal feedback; reproduction; serotonin; sex pheromone.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
Some of the data reported here were used in a patent application 62/842,072.
Figures

Comment in
-
Chemical Communication: Linking Behavior and Physiology.Curr Biol. 2019 Dec 2;29(23):R1226-R1228. doi: 10.1016/j.cub.2019.10.031. Curr Biol. 2019. PMID: 31794752 Free PMC article.
References
-
- Wyatt TD (2014). Pheromones and Animal Behavior: Chemical Signals and Signatures, 2 Edition, (Cambridge University Press; ).
-
- Aprison EZ, and Ruvinsky I (2016). Sexually Antagonistic Male Signals Manipulate Germline and Soma of C. elegans Hermaphrodites. Curr Biol 26, 2827–2833. - PubMed
-
- Aprison EZ, and Ruvinsky I (2017). Counteracting Ascarosides Act through Distinct Neurons to Determine the Sexual Identity of C. elegans Pheromones. Curr Biol 27, 2589–2599 e2583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

