Cost-utility analysis of 21-gene assay for node-positive early breast cancer
- PMID: 31708649
- PMCID: PMC6821112
- DOI: 10.3747/co.26.4769
Cost-utility analysis of 21-gene assay for node-positive early breast cancer
Abstract
Background: For women with lymph node (ln)-positive, estrogen receptor-positive, and her2 (human epidermal growth factor receptor 2)-negative breast cancer (bca), current guidelines recommend treatment with both hormonal therapy and chemotherapy. The 21-gene Recurrence Score (rs) assay might be helpful in selecting patients with bca who can be spared chemotherapy when they have 1-3 positive lns and a lower risk of recurrence. In the present study, we performed a cost-utility analysis comparing use of the 21-gene rs assay with current practice from the perspective of a Canadian health care payer.
Methods: A Markov model was developed to determine costs and quality-adjusted life-years (qalys) over a patient's lifetime. Patient outcomes in both study groups were examined based on published clinical trials. Costs were derived primarily from published Canadian sources. Costs and outcomes were discounted at 1.5% annually, and costs are reported in 2016 Canadian dollars. A probabilistic analysis was used, and the model parameters were varied in a sensitivity analysis.
Results: The results indicate that use of the 21-gene rs assay was less costly ($432 less) and more effective (0.22 qalys) than current practice. The probabilistic analysis revealed that 70% of the 10,000 simulated incremental cost-effectiveness ratios were in the southeast quadrant. The results were sensitive to the probability of a low rs and to the probability of receiving chemotherapy in the low-risk rs category and in current practice.
Conclusions: Use of the 21-gene rs assay could be a cost-effective strategy for Ontario patients with estrogen receptor-positive, her2-negative early bca and 1-3 positive lns.
Keywords: 21-gene assay; Cost-effectiveness; breast cancer; chemotherapy.
2019 Multimed Inc.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURES We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: funding for this study was received by Genomic Health Ltd. However, the research was conducted independently of Genomic Health Ltd. AE received funding from Genomic Health Ltd. for a decision study in which she was the principal investigator. ST received funding from Genomic Health Ltd. for a decision study in which she was a co-investigator and also fellowship support from Genomic Health Ltd. MT received funding from Genomic Health Ltd. for a decision study in which she was a co-investigator. IT received fees as an advisory board member for Teva Pharmaceuticals. LM, KWC, HS, and WI have no conflicts of interest to disclose.
Figures
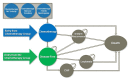
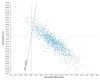

References
-
- Canadian Cancer Society. Breast Cancer Statistics [Web page] Toronto, ON: Canadian Cancer Society; n.d.. [Available at: https://www.cancer.ca/en/cancer-information/cancer-type/breast/statistic...; cited 1 January 2018]
-
- Eiermann W, Rezai M, Kümmel S, et al. The 21-gene Recurrence Score assay impacts adjuvant therapy recommendations for er-positive, node-negative and node-positive early breast cancer resulting in a risk-adapted change in chemotherapy use. Ann Oncol. 2013;24:618–24. doi: 10.1093/annonc/mds512. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

