Comparative Efficacy and Safety of Neuroprotective Therapies for Neonates With Hypoxic Ischemic Encephalopathy: A Network Meta-Analysis
- PMID: 31708771
- PMCID: PMC6824259
- DOI: 10.3389/fphar.2019.01221
Comparative Efficacy and Safety of Neuroprotective Therapies for Neonates With Hypoxic Ischemic Encephalopathy: A Network Meta-Analysis
Abstract
Context: Several interventions are available for the management of hypoxic ischemic encephalopathy (HIE), but no studies have compared their relative efficacy in a single analysis. This study aims to compare and determine the effectiveness of available interventions for HIE using direct and indirect data. Methods: Large randomized trials were identified from PubMed, EMBASE, CINAHL Plus, AMED, and Cochrane Library of Clinical Trials database from inception until June 30, 2018. Two independent reviewers extracted study data and performed quality assessment. Direct and network meta-analysis of randomized controlled trials was performed to obtained pooled results comparing the effectiveness of different therapies used in HIE on mortality, neurodevelopmental delay at 18 months, as well as adverse events. Their probability of having the highest efficacy and safety was estimated and ranked. The certainty of evidence for the primary outcomes of mortality and mortality or neurodevelopmental delay at 18 months was evaluated using GRADE criteria. Results: Fifteen studies comparing five interventions were included in the network meta-analysis. Whole body cooling [Odds ratio: 0.62 (95% credible interval: 0.46-0.83); 8 trials, high certainty of evidence] was the most effective treatment in reducing the risk of mortality, followed by selective head cooling (0.73; 0.48-1.11; 2 trials, moderate certainty of evidence) and use of magnesium sulfate (0.79; 0.20-3.06; 2 trials, low certainty of evidence). Whole body hypothermia (0.48; 0.33-0.71; 5 trials), selective head hypothermia (0.54; 0.32-0.89; 2 trials), and erythropoietin (0.36; 0.19-0.66; 2 trials) were more effective for reducing the risk of mortality and neurodevelopmental delay at 18 months (moderate to high certainty). Among neonates treated for HIE, the use of erythropoietin (0.36; 0.18-0.74, 2 trials) and whole body hypothermia (0.61; 0.45-0.83; 7 trials) were associated with lower rates of cerebral palsy. Similarly, there were lower rates of seizures among neonates treated with erythropoietin (0.35; 0.13-0.94; 1 trial) and whole body hypothermia (0.64; 0.46-0.87, 7 trials). Conclusion: The findings support current guidelines using therapeutic hypothermia in neonates with HIE. However, more trials are needed to determine the role of adjuvant therapy to hypothermia in reducing the risk of mortality and/or neurodevelopmental delay.
Keywords: hypoxic ischemic encephalopathy; meta-analysis; neonatal; neuroprotective; perinatal; systematic review.
Copyright © 2019 Lee, Chakranon and Lee.
Figures
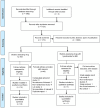
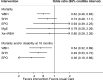
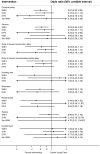
References
-
- Azzopardi D., Robertson N. J., Bainbridge A., Cady E., Charles-Edwards G., Deierl A., et al. (2016). Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-Xe): a proof-of-concept, open-label, randomised controlled trial. Lancet Neurol. 15, 145–153. 10.1016/S1474-4422(15)00347-6 - DOI - PMC - PubMed
-
- Bayley N. (1993). Bayley scales of infant and development- second edition. San Antonio, TX: Psychological Corporation.
Publication types
LinkOut - more resources
Full Text Sources

