The Therapeutic Effect of 1,8-Cineol on Pathogenic Bacteria Species Present in Chronic Rhinosinusitis
- PMID: 31708879
- PMCID: PMC6821979
- DOI: 10.3389/fmicb.2019.02325
The Therapeutic Effect of 1,8-Cineol on Pathogenic Bacteria Species Present in Chronic Rhinosinusitis
Abstract
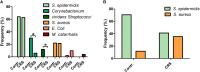
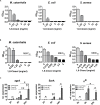
Chronic rhinosinusitis (CRS) is marked by an inflamed mucosa of sinuses and is accompanied by a significantly reduced quality of live. Since no guidelines for the treatment of CRS are available, long lasting clinical histories with health care costs adding up to dozens of billion $ annually are caused by CRS. The progression of CRS is often induced by bacterial infections and/or a shift in microbiome as well as biofilm formation. The exact microbiome alterations are still unclear and the impenetrable biofilm renders the treatment with common antibiotics ineffective. This study focuses on characterizing the microbiome changes in CRS and investigating the inhibition of biofilm growth by 1,8-Cineol, a small, non-polar and hence biofilm penetrating molecule with known antimicrobial potential. We performed MALDI-TOF MS based characterization of the microbiomes of healthy individuals and CRS patients (n = 50). The microbiome in our test group was shifted to pathogens (Staphylococcus aureus, Escherichia coli, and Moraxella catarrhalis). In contrast to published studies, solely based on cell culture techniques, we could not verify the abundance of Pseudomonas aeruginosa in CRS. The inhibition of bacterial proliferation and biofilm growth by 1,8-Cineol was measured for these three pathogens. Interestingly, S. aureus, the most prominent germ in CRS, showed a biofilm inhibition not simply correlated to its inhibition of proliferation. RT-qPCR confirmed that this was due to the downregulations of major key players in biofilm generation (agrA, SarA and σB) by 1,8-Cineol. Furthermore we verified this high biofilm inhibition potential in a model host system consisting out of S. aureus biofilm grown on mature respiratory epithelium. A second host model, comprising organotypic slices, was utilized to investigate the reaction of the innate immune system present in the nasal mucosa upon biofilm formation and treatment with 1,8-Cineol. Interestingly Staphylococcus epidermidis, the cause of very common catheter infections, possesses a biofilm generation pathway very similar to S. aureus and might be treatable in a similar fashion. The two presented in vitro model systems might be transferred to combinations of every biofilm forming bacterial with most kind of epithelium and mucosa.
Keywords: 1; 8-Cineol; S. aureus; biofilm; chronic rhinosinusitis; host model system.
Copyright © 2019 Schürmann, Oppel, Gottschalk, Büker, Jantos, Knabbe, Hütten, Kaltschmidt, Kaltschmidt and Sudhoff.
Figures

References
-
- Bakkali F., Averbeck S., Averbeck D., Idaomar M. (2008). Biological effects of essential oils–a review. Food Chem. Toxicol. 46 446–475. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

