The Diverse Functional Roles of Elongation Factor Tu (EF-Tu) in Microbial Pathogenesis
- PMID: 31708880
- PMCID: PMC6822514
- DOI: 10.3389/fmicb.2019.02351
The Diverse Functional Roles of Elongation Factor Tu (EF-Tu) in Microbial Pathogenesis
Abstract
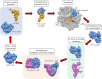
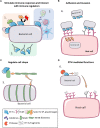
Elongation factor thermal unstable Tu (EF-Tu) is a G protein that catalyzes the binding of aminoacyl-tRNA to the A-site of the ribosome inside living cells. Structural and biochemical studies have described the complex interactions needed to effect canonical function. However, EF-Tu has evolved the capacity to execute diverse functions on the extracellular surface of both eukaryote and prokaryote cells. EF-Tu can traffic to, and is retained on, cell surfaces where can interact with membrane receptors and with extracellular matrix on the surface of plant and animal cells. Our structural studies indicate that short linear motifs (SLiMs) in surface exposed, non-conserved regions of the molecule may play a key role in the moonlighting functions ascribed to this ancient, highly abundant protein. Here we explore the diverse moonlighting functions relating to pathogenesis of EF-Tu in bacteria and examine putative SLiMs on surface-exposed regions of the molecule.
Keywords: EF-Tu; adhesion; bacteria; chaperone activities; moonlighting.
Copyright © 2019 Harvey, Jarocki, Charles and Djordjevic.
Figures


Similar articles
-
Structural homology between elongation factors EF-Tu from Bacillus stearothermophilus and Escherichia coli in the binding site for aminoacyl-tRNA.Eur J Biochem. 1986 Jan 15;154(2):355-62. doi: 10.1111/j.1432-1033.1986.tb09405.x. Eur J Biochem. 1986. PMID: 3510872
-
Mutagenesis of Arg335 in bovine mitochondrial elongation factor Tu and the corresponding residue in the Escherichia coli factor affects interactions with mitochondrial aminoacyl-tRNAs.RNA Biol. 2004 Jul;1(2):95-102. doi: 10.4161/rna.1.2.1034. Epub 2004 Jul 16. RNA Biol. 2004. PMID: 17179748
-
Fluorescence characterization of the interaction of various transfer RNA species with elongation factor Tu.GTP: evidence for a new functional role for elongation factor Tu in protein biosynthesis.Biochemistry. 1990 May 8;29(18):4268-77. doi: 10.1021/bi00470a002. Biochemistry. 1990. PMID: 2190631
-
tRNA-ribosome interactions.Biochem Cell Biol. 1995 Nov-Dec;73(11-12):1049-54. doi: 10.1139/o95-112. Biochem Cell Biol. 1995. PMID: 8722020 Review.
-
Elongation factor Tu, a GTPase triggered by codon recognition on the ribosome: mechanism and GTP consumption.Biochem Cell Biol. 1995 Nov-Dec;73(11-12):1221-7. doi: 10.1139/o95-132. Biochem Cell Biol. 1995. PMID: 8722040 Review.
Cited by
-
Probiotic Gastrointestinal Transit and Colonization After Oral Administration: A Long Journey.Front Cell Infect Microbiol. 2021 Mar 10;11:609722. doi: 10.3389/fcimb.2021.609722. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33791234 Free PMC article. Review.
-
Enrichment of oral-derived bacteria in inflamed colorectal tumors and distinct associations of Fusobacterium in the mesenchymal subtype.Cell Rep Med. 2023 Feb 21;4(2):100920. doi: 10.1016/j.xcrm.2023.100920. Epub 2023 Jan 26. Cell Rep Med. 2023. PMID: 36706753 Free PMC article.
-
The Influence of Symbiosis on the Proteome of the Exaiptasia Endosymbiont Breviolum minutum.Microorganisms. 2023 Jan 22;11(2):292. doi: 10.3390/microorganisms11020292. Microorganisms. 2023. PMID: 36838257 Free PMC article.
-
Biofilms and core pathogens shape the tumor microenvironment and immune phenotype in colorectal cancer.Gut Microbes. 2024 Jan-Dec;16(1):2350156. doi: 10.1080/19490976.2024.2350156. Epub 2024 May 10. Gut Microbes. 2024. PMID: 38726597 Free PMC article.
-
Grape polyphenols reduce fasting glucose and increase hyocholic acid in healthy humans: a meta-omics study.NPJ Sci Food. 2025 May 27;9(1):87. doi: 10.1038/s41538-025-00443-6. NPJ Sci Food. 2025. PMID: 40425565 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

