Pivotal Role of Mitochondria in Macrophage Response to Bacterial Pathogens
- PMID: 31708919
- PMCID: PMC6819784
- DOI: 10.3389/fimmu.2019.02461
Pivotal Role of Mitochondria in Macrophage Response to Bacterial Pathogens
Abstract
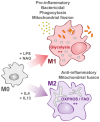
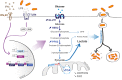
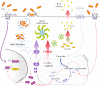
Mitochondria are essential organelles that act as metabolic hubs and signaling platforms within the cell. Numerous mitochondrial functions, including energy metabolism, lipid synthesis, and autophagy regulation, are intimately linked to mitochondrial dynamics, which is shaped by ongoing fusion and fission events. Recently, several intracellular bacterial pathogens have been shown to modulate mitochondrial functions to maintain their replicative niche. Through selected examples of human bacterial pathogens, we will discuss how infection induces mitochondrial changes in infected macrophages, triggering modifications of the host metabolism that lead to important immunological reprogramming.
Keywords: bacterial infection; cell polarization; immunometabolism; macrophage; mitochondria.
Copyright © 2019 Ramond, Jamet, Coureuil and Charbit.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

