Pregnancy-Induced Alterations in NK Cell Phenotype and Function
- PMID: 31708922
- PMCID: PMC6820503
- DOI: 10.3389/fimmu.2019.02469
Pregnancy-Induced Alterations in NK Cell Phenotype and Function
Abstract
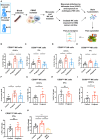
Pregnant women are particularly susceptible to complications of influenza A virus infection, which may result from pregnancy-induced changes in the function of immune cells, including natural killer (NK) cells. To better understand NK cell function during pregnancy, we assessed the ability of the two main subsets of NK cells, CD56dim, and CD56bright NK cells, to respond to influenza-virus infected cells and tumor cells. During pregnancy, CD56dim and CD56bright NK cells displayed enhanced functional responses to both infected and tumor cells, with increased expression of degranulation markers and elevated frequency of NK cells producing IFN-γ. To better understand the mechanisms driving this enhanced function, we profiled CD56dim and CD56bright NK cells from pregnant and non-pregnant women using mass cytometry. NK cells from pregnant women displayed significantly increased expression of several functional and activation markers such as CD38 on both subsets and NKp46 on CD56dim NK cells. NK cells also displayed diminished expression of the chemokine receptor CXCR3 during pregnancy. Overall, these data demonstrate that functional and phenotypic shifts occur in NK cells during pregnancy that can influence the magnitude of the immune response to both infections and tumors.
Keywords: NK cells; NK repertoire; cancer cells; influenza virus; pregnancy.
Copyright © 2019 Le Gars, Seiler, Kay, Bayless, Starosvetsky, Moore, Shen-Orr, Aziz, Khatri, Dekker, Swan, Davis, Holmes and Blish.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

