Evaluation of human primary intestinal monolayers for drug metabolizing capabilities
- PMID: 31709009
- PMCID: PMC6829970
- DOI: 10.1186/s13036-019-0212-1
Evaluation of human primary intestinal monolayers for drug metabolizing capabilities
Abstract
Background: The intestinal epithelium is a major site of drug metabolism in the human body, possessing enterocytes that house brush border enzymes and phase I and II drug metabolizing enzymes (DMEs). The enterocytes are supported by a porous extracellular matrix (ECM) that enables proper cell adhesion and function of brush border enzymes, such as alkaline phosphatase (ALP) and alanyl aminopeptidase (AAP), phase I DMEs that convert a parent drug to a more polar metabolite by introducing or unmasking a functional group, and phase II DMEs that form a covalent conjugate between a functional group on the parent compound or sequential metabolism of phase I metabolite. In our effort to develop an in vitro intestinal epithelium model, we investigate the impact of two previously described simple and customizable scaffolding systems, a gradient cross-linked scaffold and a conventional scaffold, on the ability of intestinal epithelial cells to produce drug metabolizing proteins as well as to metabolize exogenously added compounds. While the scaffolding systems possess a range of differences, they are most distinguished by their stiffness with the gradient cross-linked scaffold possessing a stiffness similar to that found in the in vivo intestine, while the conventional scaffold possesses a stiffness several orders of magnitude greater than that found in vivo.
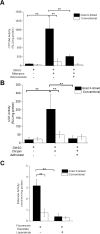
Results: The monolayers on the gradient cross-linked scaffold expressed CYP3A4, UGTs 2B17, 1A1 and 1A10, and CES2 proteins at a level similar to that in fresh crypts/villi. The monolayers on the conventional scaffold expressed similar levels of CYP3A4 and UGTs 1A1 and 1A10 DMEs to that found in fresh crypts/villi but significantly decreased expression of UGT2B17 and CES2 proteins. The activity of CYP3A4 and UGTs 1A1 and 1A10 was inducible in cells on the gradient cross-linked scaffold when the cells were treated with known inducers, whereas the CYP3A4 and UGT activities were not inducible in cells grown on the conventional scaffold. Both monolayers demonstrate esterase activity but the activity measured in cells on the conventional scaffold could not be inhibited with a known CES2 inhibitor. Both monolayer culture systems displayed similar ALP and AAP brush border enzyme activity. When cells on the conventional scaffold were incubated with a yes-associated protein (YAP) inhibitor, CYP3A4 activity was greatly enhanced suggesting that mechano-transduction signaling can modulate drug metabolizing enzymes.
Conclusions: The use of a cross-linked hydrogel scaffold for expansion and differentiation of primary human intestinal stem cells dramatically impacts the induction of CYP3A4 and maintenance of UGT and CES drug metabolizing enzymes in vitro making this a superior substrate for enterocyte culture in DME studies. This work highlights the influence of mechanical properties of the culture substrate on protein expression and the activity of drug metabolizing enzymes as a critical factor in developing accurate assay protocols for pharmacokinetic studies using primary intestinal cells.
Keywords: CYP3A4; Drug metabolism; Extracellular matrix; Small intestine; Stiffness.
© The Author(s). 2019.
Conflict of interest statement
Competing interestsN.L.A. and Y.W. have a financial interest in Altis Biosystems, Inc. The remaining authors disclose no conflicts.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

